Translate this page into:
Why internal carotid artery pulsations are not audible?

*Corresponding author: Mustafa Ismail, Department of Neurosurgery, Medical University of South Carolina, Charleston, South Carolina, United States. mustafalorance2233@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Abdulkareem Saleh S, Hammoud Z, Farhat S, Abdulhameed MA, Ismail M, Hoz SS. Why internal carotid artery pulsations are not audible? Adesh Univ J Med Sci Res. 2024;6:74-8. doi: 10.25259/AUJMSR_37_2023
Abstract
Background:
Arterial pulsation, the force ensuing from swift blood ejection into the aorta and its subsequent transmission across the arterial system, leads to an abrupt expansion of the arterial wall. Perceiving the rhythmic whoosh of one’s own internal carotid artery (ICA) pulse in synchronization with the heartbeat is conventionally improbable. In this review, we delve into the intricate physiological and anatomical underpinnings of the auditory apparatus, scrutinize the factors precluding the audibility of the ICA pulsation, and highlight the potential clinical implications of perceptible ICA pulsations and associated pathologies, which could significantly impact patient care.
Material and Methods:
We conducted a literature review in PubMed and Google Scholar databases to review the existing literature describing the relationship between ICA pulsations and hearing apparatus.
Results:
The literature review did not yield papers targeting the hearing of carotid artery pulsation. However, we used the available indirectly related articles to formulate an overview to serve the aim of this paper and to highlight the potential value of studying the audible carotid artery and its pathologies. This research is important as it could provide a deeper understanding of the relationship between arterial pulsations and the auditory system.
Conclusion:
Three pivotal factors could contribute to the carotid pulse’s inaudibility: The petrous carotid canal’s sound-dampening effect, the pulse’s frequency below the human ear’s most detectable range, and the dampening role of the soft tissues around the ICA, including the pericarotid venous plexus.
Keywords
Carotid artery
Carotid pulse
Hearing physiology
Internal carotid artery
Petrous bone
INTRODUCTION
Arterial pulsation is typified by the propulsive energy generated from the swift propulsion of blood into the aorta and its subsequent distribution across the arterial system. This kinetic activity induces an immediate distention of the arterial wall, achieving its zenith in tandem with systolic blood pressure.[1] Progressing from the central aorta toward the peripheral arteries, this arterial pulse undergoes modifications due to the surge in systolic pressure, thereby augmenting its force. Consequently, an in-depth assessment of the carotid artery, bearing the most proximal arterial pulse, is imperative to garner information concerning the contour of the arterial pulse. This is particularly pertinent given the multitude of factors influencing the transmission of the pulse throughout the arterial network.
Nevertheless, although the rhythmic pulsations can be palpated, the auditory perception of one’s pulse, synchronously resounding with the heartbeat, is generally unattainable. Owing to a myriad of causative factors, this review will delve into the intricacies of auditory physiology and anatomy, investigating the reasons that preclude the audibility of carotid artery pulsations. Furthermore, we will explore the clinical ramifications of perceptible carotid pulsations, in addition to associated pathological conditions.
MATERIAL AND METHODS
A comprehensive exploration of scientific articles was carried out, utilizing the PubMed and Google Scholar databases. The focus of this review was to discern available literature elucidating the interrelationship between the pulsations of the internal carotid artery (ICA) and auditory structures.
RESULTS
Our literature survey failed to identify publications expressly addressing the audibility of carotid artery pulsations. Nevertheless, utilizing the accessible literature bearing indirect relevance, we synthesized an overview to fulfill the objective of this article. Moreover, we underscored the potential importance of research focusing on the audibility of carotid artery pulsations and associated pathologies.
DISCUSSION
The hearing apparatus
Through the detection of oscillations, which are recurring variations in the surrounding medium’s pressure, the auditory apparatus allows humans to perceive sounds. Each of the three physical organs that make up the ear – the middle, inner, and outer – plays a crucial part in converting these external waves into audible sounds.
Anatomy of the outer, middle, and inner ear
Sound waves from the environment first reach the outer ear, which includes structures such as the pinna, external auditory canal, and tympanic membrane. Behind this lies the middle ear, an air-filled cavity that holds three tiny bones – called the malleus, incus, and stapes – that help transmit sound vibrations from the eardrum to the inner ear. The stapes press against the oval window of the cochlea.[2]
The inner ear contains the cochlea, semicircular canals, utricle, and saccule. These structures are situated within the dense portion of the temporal bone. Unlike the surrounding bony labyrinth, they have distinct functions in hearing and balance.[2]
The cochlea, semicircular canals, utricle, and saccule are all parts of the inner ear, which is located inside the petrous part of the temporal bone. Different from the bony labyrinth, these elements make up the membrane labyrinth. Endolymph, which is present in the membranous labyrinth, is essential for stimulating the hair cells that convey vestibular and auditory signals.[3] The inner ear receives its innervation from the vestibulocochlear nerve (cranial nerve eight [CN VIII]). The vestibular component, responsible for balance, and the cochlear nerve, governing hearing, enter the inner ear along with the labyrinthine artery through the internal acoustic meatus. Despite traversing the inner ear, the facial nerve, or CN VII, lacks any innervation within.[3]
The cochlea is a coiled, fluid-filled organ located in the cochlear duct. It contains three compartments: the scala vestibuli, scala media (also known as the cochlear duct), and scala tympani. The scala media, filled with endolymph, is positioned between the scala vestibuli and scala tympani, which are both filled with perilymph. Endolymph is secreted by the stria vascularis, a dense capillary network in the spiral ligament, and is derived from cerebrospinal fluid. Perilymph in the scala tympani originates from cerebrospinal fluid, while the scala vestibuli’s perilymph comes from blood plasma.[2]
The cochlea functions are based on stark differences in ion concentrations between endolymph and perilymph. Endolymph is rich in potassium, whereas perilymph contains more sodium and relatively less potassium and calcium. These ionic imbalances create the endocochlear potential, which is essential for proper electrical signaling within the hair cells.[2]
The physiology of hearing
The human auditory range typically spans from 20 to 20,000 hertz, with personal variation. On a decibel scale, which measures sound volume, the auditory range extends from 0 to 130 dB, where sound becomes distressing. To be processed by the central nervous system, these physical phenomena must be transformed. Initially, air vibrations are transmuted into tympanic membrane oscillations. Subsequently, these oscillations affect the middle ear and ossicles, further transmuting into liquid vibrations within the cochlea and inner ear. This motion activates both the basilar membrane and the organ of Corti, leading to the production of nerve signals that are relayed to the brain.[4]
Clear cells, believed to regulate the inner ear’s ionic environment, vital for proper auditory function, reside in the stria vascularis – a highly vascularized tissue lining the cochlea’s outer wall, the site of endolymph production. Immersed in this fluid are the inner ear’s sensory cells that detect sound vibrations. Clear cells are postulated to maintain the endolymph’s high potassium concentration, crucial for hair cell functionality, regulate the endolymph’s pH, and remove inner ear waste products.[5]
The anatomy of the carotid artery
The carotid artery is a major cervical blood vessel responsible for supplying oxygen-rich blood to the brain, face, and neck. As it travels upward, it divides into two main branches: The internal and external carotid arteries. The external carotid artery gives rise to numerous branches that deliver blood to regions of the scalp, face, and neck. These branches include the superior thyroid, occipital, facial, lingual, pharyngeal, posterior auricular, maxillary, and superficial temporal arteries.
The ICA, on the other hand, ascends through the neck and may enter the skull through the carotid canal. Once it reaches the cranial cavity, it provides blood to critical brain structures, including through the anterior and middle cerebral arteries. Based on Bouthillier’s classification, the ICA is divided into seven anatomical segments. The first segment, known as the cervical artery (C1), stretches from the point where the common carotid artery divides up to the opening of the carotid canal. The second segment, the petrous artery (C2), continues from this opening to the back edge of the foramen lacerum. The lacerum artery (C3) extends from the top of the petrolingual ligament to the posterior boundary of the foramen lacerum. The fourth segment called the cavernous artery (C4), runs from the proximal dural ring to the upper part of the petrolingual ligament near the anterior clinoid process. The clinoid artery (C5) lies between the anterior clinoid process and the distal dural ring at the roof of the cavernous sinus. The sixth segment, the ophthalmic artery (C6), starts just before the origin of the posterior connecting artery and continues through the distal dural ring. Finally, the communicating artery (C7) runs from the internal carotid bifurcation to the point where the posterior communicating artery begins.[6]
In terms of the anatomical and physiological relationship between auditory perception and the ICA’s petrous part, the petrous part is embedded within the carotid canal, which ascends virtually vertically through the petrous temporal bone segment. Initially, this artery segment passes medially to the cochlea, then it proceeds forward and laterally, forming the carotid genu (hairpin turn), then it reverses direction to penetrate the dura mater, above the glossopharyngeal nerve’s origin. It then follows the cavernous sinus’ lateral wall. Although the petrous part of the ICA does not directly supply blood to the auditory organs, its proximity to the vestibular apparatus and cochlea could potentially influence these auditory structures in case of arterial pathology or injury.[7]
Pulsation of the carotid artery
Pulsation is a term that refers to the regular expansion and contraction of the artery as the heart pumps blood into it as well as it may be used to describe the heart’s regular beating. In fact, every time the heart beats, a pressure wave is created and travels within the arteries, where it is felt as a pulsation in some arteries in the body like the carotid artery. Normally, the sound of blood flowing through arteries is too soft for the human ear to perceive therefore we are unable to hear our pulse. Since blood flow produces low-frequency sound waves, other environmental sounds frequently drown them out. The sound of the heartbeat is also not audible to the human ear without the use of medical equipment like a stethoscope because the human ear is not sensitive enough to detect the minute vibrations produced by the flow of blood.[8]
Using a stethoscope, which amplifies the noises and makes it possible to hear the pulsation of the heartbeat, has made it feasible to hear the pulsation since hearing and experiencing the pulsation is highly crucial as it reflects important information on physical health. Alternatively, the carotid artery in the neck, which is closer to the skin, can be felt to detect the pulsation. The artery is placed between the index and middle fingers, which are then gently pressed down until a pulsing feeling is felt.[9]
Dissecting inaudibility: Understanding the carotid artery pulsation
There are potential causes that may explain why carotid artery pulsation is inaudible. One of the reasons could be due to carotid artery location and separation: The carotid canal, which is located inside the temporal bone and connected to the cochlea by the bony labyrinth of the inner ear, serves as the petrous portion’s pathway to act as an effective barrier to muffle the sounds produced by the ICA, rendering them inaudible to the human ear. Similar to this, the bony walls of the temporal bone separate the ICA from the vestibular system and the auditory and vestibular nerves.[7,10]
The anterior-inferior portion of the carotid canal, that is relatively close to the cochlear twists, is where the chloroform process changes direction and begins its horizontal segment from an anatomical standpoint. The carotid canal and cochlea are, therefore most usually seen at the basal turn, less frequently in the medium turn, and less often in the apical turn. It has been reported that the bone thickness between the carotid canal and the otic capsule varies between 0.5 and 10 mm. Consequently, at its closest point, the carotid canal was, on average, 1.05 mm from the cochlea [Figure 1].[7] Because the size and structure of the bone labyrinth vary depending on a number of factors, including an individual’s age, anatomy, and general health, the distance that exists between the ICA and the hearing apparatus may also vary.
In fact, the cochlea is situated superior to and posterior to the petrous carotid canal in the anterior part of the otic capsule. The diameter of the bony cochlear canal shrinks as the diameter of the basilar membrane increases from base to peak. With their highest low-frequency resonance at the apex (20 kHz and 20 Hz, each) and their maximum high-frequency resonance at the base, the hair cells on the basilar membrane exhibit a wide dynamic frequency range. The mid-tone frequencies around 1 and 5 kHz are primarily controlled by the basal turn.[11]
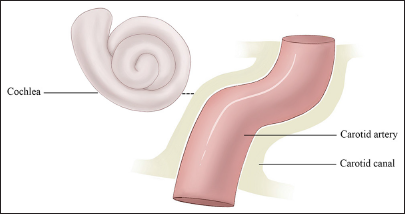
- This figure demonstrates that the cochlea orientation in relation to the carotid canal and the closest distance between the cochlea and the carotid canal is 1.05 mm on average.
The human ear is sensitive to sounds between 2000 and 5000 hertz, which is the range of frequencies where speech sounds are most prominent. The pulsations of the ICA typically produce sounds with a frequency range of 500–1000 hertz, which is below the range of frequencies that are most audible to the human ear.[12] Even though, these sound waves could be dampened and muffled by the layers of soft tissue, including muscle, fat, and connective tissue, that surround the artery. These layers may act as a barrier, blocking the sound waves from reaching the surface of the skin where they can be heard. For example, the pericarotid venous plexus which is close to the cochlea side might suppress the carotid vibration by acting as a sound barrier. This is confirmed in cases of glomus tumor and dural fistula where this damping mechanism is lost and the pulse becomes audible.[13] The bony labyrinth is made up mostly of solid bone, however, it is generally quite thin. The ICA is surrounded by the petrous bone, which is a portion of the temporal bone. As the bone grows toward the cochlea and other inner ear tissues, the thickness of the bony walls becomes thinner.
Clinical implications of audible carotid pulsation
Several proposed mechanisms may underlie the perception of carotid pulse sounds. One such mechanism could involve vessel stenosis or the transmission of turbulence or high-velocity blood flow sound waves to the inner ear, producing noise. Another potential mechanism implicates conductive changes within the ossicular chain, causing the sound of normal blood flow to be perceived as louder than usual. A variety of pathologies could potentially affect the ICA, resulting in an audible pulse. These include atherosclerotic ICA stenosis, which can lead to compensatory accelerated blood flow in the contralateral open vessel, resulting in a pronounced pulse. The convoluted carotid artery, carotid-cavernous sinus fistula resulting from a fracture of the skull base, fibromuscular dysplasia of the ICA, aneurysm or dissection of the petrous segment of the ipsilateral ICA, and vascular diseases of the petrous bone along with skull base may also be affected. Due to turbulent blood flow, that is then transmitted to the cochlea, such circumstances typically result in noise.[14]
Pulsation may be sent straight to the cochlea through the internal auditory canal when a vascular loop penetrates the internal auditory meatus.[13] Other potential causes could include dural arteriovenous malformations or fistulas, glomus tumors, carotid-cochlear dehiscence, aberrant ICAs, and vessel-rich tumors at the skull base, such as temporal bone hemangiomas.[15]
Given the potential for the audible perception of carotid pulse to indicate a serious condition, it is paramount to conduct thorough investigations to ascertain the underlying cause and establish appropriate management strategies.
CONCLUSION
Three key factors could account for the typical inaudibility of the carotid pulse. First, the carotid canal’s petrous portion effectively muffles pulse-generated sound waves. Second, the frequency of these waves is typically below the human ear’s most detectable range. Finally, soft tissues around the petrous bone’s ICA, including the pericarotid venous plexus, help to dampen pulse vibrations, preventing their transmission to the cochlea.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Clinical methods: The history, physical, and laboratory examinations. Vol 2. Boston: Butterworths; 1990.
- [Google Scholar]
- Morphology of human ear canal and its effect on sound transmission. Int J Numer Method Biomed Eng. 2022;38:e3567.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy and surgical approach of the ear and temporal bone. Head Neck Pathol. 2018;12:321-7.
- [CrossRef] [PubMed] [Google Scholar]
- Modeling of sound transmission from ear canal to cochlea. Ann Biomed Eng. 2007;35:2180-95.
- [CrossRef] [PubMed] [Google Scholar]
- Functional structure of the organ of Corti: A review. Hear Res. 1986;22:117-46.
- [CrossRef] [PubMed] [Google Scholar]
- Segments of the internal carotid artery: A new classification. Neurosurgery. 1996;38:425-32.
- [CrossRef] [PubMed] [Google Scholar]
- Microscopic anatomy of the carotid canal and its relations with cochlea and middle ear. Braz J Otorhinolaryngol. 2005;71:410-4.
- [CrossRef] [PubMed] [Google Scholar]
- Bedside cardiac examination: Constancy in a sea of change. Curr Probl Cardiol. 2000;25:783-825.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomic assessment of petrous internal carotid artery, facial nerve, and cochlea through the anterior transpetrosal approach. J Craniofac Surg. 2015;26:2180-3.
- [CrossRef] [PubMed] [Google Scholar]
- Auditory pathways: Anatomy and physiology. Handb Clin Neurol. 2015;129:3-25.
- [CrossRef] [PubMed] [Google Scholar]
- The evaluation of the sense of hearing in patients with carotid artery stenosis within the extracranial segments. Acta Neurol Belg. 2019;119:385-92.
- [CrossRef] [PubMed] [Google Scholar]
- Pulsatile tinnitus and the intrameatal vascular loop: Why do we not hear our carotids? Neurosurgery. 2005;57:1213-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pulsatile tinnitus associated with internal carotid artery morphologic abnormalities. Otol Neurotol. 2008;29:1032-6.
- [CrossRef] [PubMed] [Google Scholar]







