Translate this page into:
Treatment with Markhamia tomentosa Benth. K. Schum prevents carbon tetrachloride-induced liver damage in rats

*Corresponding author: Romeo Joel Guemmogne Temdie, Department of Biological Sciences, University of Ngaoundere, Ngaoundere, Cameroon. temdie2011@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Temdie RJ, Jidibe P, Galani BR, Vouffo EY, Djasrane AD, Boumzina EL, et al. Treatment with Markhamia tomentosa Benth. K. Schum prevents carbon tetrachloride-induced liver damage in rats. Adesh Univ J Med Sci Res 2022;4:94-104.
Abstract
Objective:
Markhamia tomentosa (Bignoniaceae) is a medicinal plant with several pharmacological properties. However, its hepatoprotective effects have been little studied. The aim of this study was to evaluate the protective effects of the aqueous trunk bark extract of this plant against carbon tetrachloride (CCl4)-induced liver injury in rat.
Material and Methods:
Thirty male albino Wistar rats were divided into six groups (five each) with Groups 1, 2, 3, and 4 as negative (distilled water), normal (distilled water), positive (silymarin 25 mg/kg), and plant extract (50 mg/kg) controls, respectively. Groups 5 and 6 were used as test groups and were given plant extract (25 or 50 mg/kg, respectively). Rats were pretreated once a day for 14 days orally with different substances. CCl4 (0.5 mL/kg, i.p.) was administered on days 4 and 11 to all groups except Groups 1 and 4, to induce hepatitis. The rats were then sacrificed on day 15; liver functions and oxidative stress were assessed as well as histopathological changes.
Results:
M. tomentosa extract significantly and dose dependently decreased alanine aminotransferase, aspartate aminotransferase, gamma-glutamine aminotransferase, total bilirubin, total cholesterol, and malondialdehyde values while increasing catalase, and glutathione values compared to the CCl4-treated group. Histological findings showed a reduction in necrosis and inflammatory cell infiltration in the liver while the lumen of distal and proximal tubes was improved in the kidney by the plant extract. These results may be due to some of the major bioactives compounds found in the aqueous extract.
Conclusion:
These findings suggest that the aqueous extract of M. tomentosa may have liver protective effects through its antioxidant and anti-inflammatory mechanisms, supporting thereby its ethnomedicinal uses.
Keywords
Liver diseases
Markhamia tomentosa
Hepatoprotective activity
Carbone tetrachloride
Antioxidant effect
INTRODUCTION
The liver is a major organ involved in the metabolism of endogenous or exogenous substances.[1,2] This metabolic activity exposes the liver cells to the toxicity of xenobiotic and their metabolites and thereby increases the risk of causing injury to liver, leading to liver inflammation. Hepatitis, an inflammation of the liver tissue, can also be caused by infectious agents (bacterial, virus, and parasites) or toxins that damage the liver cells.[3,4] During the process of liver injury, intracellular substances leak out of injured cells, leading to the activation of neutrophils and monocytes, and massive recruitment of these immune cells into the damaged liver tissue. Inflammatory cells are known to actively promote the production of reactive species, tumor necrosis factor-alpha (TNF-α), and other pro-inflammatory cytokines that are involved in the worsening of tissue lesions.[5]
Carbon tetrachloride (CCl4) is one of the chemical products frequently used to induce liver injury.[6] The toxicity of CCl4 results from its metabolism by cytochrome P450 in the highly reactive species, trichloromethyl radical (CCl3), and trichloromethyl peroxy radical (CCl3OO).[7] These molecules attack polyunsaturated fatty acids of biological membranes and cause lipid peroxidation, which contributes to severe cellular damages.[8,9] Destruction of mitochondria membrane by CCl4 metabolites triggers caspase-3-dependent apoptosis through specific cleavage of key cellular proteins by caspase-3, a major cell death proteases.[10] It has been proposed that the primary way to protect against CCl4-induced liver damage is to inhibit the production of free radicals.[11]
Medicinal plants have been used for centuries to treat diseases due to their safety and efficacy, as well as their richness in substances that could be used for therapeutic purposes or as precursors to the synthesis of useful drugs.[12] In this adventure, no part of the plant is left unexplored. Stem, roots, leaves, flowers, fruits, bark, and wood are all studied for their medicinal potential.
Markhamia tomentosa is a medicinal plant of the Bignoniaceae family found mainly in tropical regions. [13] Ethnopharmacological data indicate its use against headaches, canker, lumbago, chest pain, edema, rheumatic pain, scrotal elephantiasis, anemia, diarrhea, backache, sore eyes, intercostal pain, snakebite/venom, ailments of the reproductive system, lung disorders, gout, bouts of swamp fever, and external skin diseases.[14-17] A study revealed the presence of alkaloids, tannins, saponins, anthraquinones, cardiac glycosides, glycosides, phenols, and flavonoids in M. tomentosa methanol leaf extract and two compounds, namely, 2-acetyl-6-methoxynaphtho[2,3-b] furan-4,9-dione and 2-acetylnaphtho[2,3-b]furan-4,9-dione were identified in the ethyl acetate leaf extract.[18,19] Based on these usages and probably on phytochemical composition, the previous studies demonstrated that M. tomentosa possessed antiprotozoal and larvicidal activities, analgesic effects, acute and chronic anti-inflammatory effects, and antiarthritic effects.[18-22] It was also proved that aqueous and methanol leaf extract of M. tomentosa was relatively non-toxic to rats.[17] More recently, it was found that the methanol leaf extract of M. tomentosa exhibited a protective effect on D-galactosamine/ lipopolysaccharide-induced acute hepatitis.[23] However, to the best of our knowledge, no research on the CCl4-induced liver damage has been conducted. Furthermore, the search for a viable natural remedy for liver diseases led us to investigate the potential protective benefits of aqueous extract of M. tomentosa stem bark against CCl4-induced liver injury in rats.
MATERIAL AND METHODS
Plant material
M. tomentosa bark was collected in July 2019, in the locality of Bayangam, West region-Cameroon. The identification was made by botanist of the National Herbarium of Cameroon, in comparison with an existing voucher specimen registered under the number 1974/SRFK. The harvested bark was cut into small pieces that were dried in the shade. The dried bark was crushed with an electric grinder and sieved, and the powder was carefully stored in a plastic bottle for extraction.
Preparation of the aqueous extract
The plant extract was prepared as previously described by Temdie et al.[19] Briefly, 150 g of the M. tomentosa trunk bark powder was introduced into the adiabatic device with 750 mL of hot distilled water (60°C) and the mixture was stirred regularly for 24 h. The preparation was decanted and filtered through Whatman No. 3 paper. Two milliliters of the filtrate were evaporated in an oven to determine the extraction concentration (28 mg/mL). The remaining filtrate was stored at −20°C for later use.
Experimental animals
The study was conducted on male albino rats weighing 150 ± 10 g. These rats were reared under natural conditions (room temperature, 12 h/12 h photoperiod, and average humidity of 79 ± 10%) with free access to water and standard food. The research was conducted in accordance with the revised protocols for the care of laboratory animals (NIH publication No. 85-23, 1985) and the authorization granted by the Cameroon National Ethical Committee (Reg. No. FWAIRD 0001954).
Qualitative phytochemicals analysis
Qualitative phytochemical analysis of the aqueous extract of M. tomentosa bark was performed to look for compounds such as polyphenols, flavonoids, tannins, anthraquinones, saponins, anthocyanins, triterpenes, and alkaloids following the protocol previously described by N’guessan et al.[24]
Experimental design
Thirty rats were divided into six groups of five rats each made up of four control groups (healthy, hepatitis, extract, and positive control groups) and two test groups according to treatment received [Table 1]. The animals were weighed on the 1st day before treatment and then daily. Distilled water, silymarin (Micro Labs Limited, Bengaluru, India), and plant extract at the doses of 25 or 50 mg/kg were administered orally (10 mL/kg) at a daily dose for 14 days. Liver injury was induced after 12 h of fasting by intraperitoneal injection of a mixture of an equal volume (1/1) of carbon tetrachloride (Sigma-Aldrich, St. Louis, Missouri, USA) and olive oil (Lesieur, 29, Quai Aulagnier 92665 Sur Seine Cedex, France) at the dose of 0.5 mL/kg, on days 4 and 11, except for the healthy and extract control groups, which received an equivalent volume of olive oil in the same manner.[25] At the end of the treatment, the rats were sacrificed under anesthesia (ethylic ether) by decapitation. The blood sample was collected in dry tubes and centrifuged at 3000 rpm for 15 min to obtain serum. Liver and kidney were removed, rinsed in normal saline solution, and weighed. The liver and kidney sample was cold ground in Tris-HCl buffer (50 mM, pH 7.4) to obtain a homogenate (20%), which was centrifuged at 3000 rpm for 15 min. The supernatant was recovered and stored as well as the serum at −20°C for the biochemical analysis. Another sample of liver and kidney was submerged in buffered formalin (10%) for histological analysis.[23]
| Selected animals | 30 rats | |||||
|---|---|---|---|---|---|---|
| Groups formed | Hepatitis control | Healthy control | Positive control | Extract control | Test groups | |
| Treatment received | H2O+CCl4 | H2O+Olive oil | Sily 25+CCl4 | ATBE 50+Olive oil | ATBE 50+CCl4 | ATBE 25+CCl4 |
Sily 25: Silymarin at a dose of 25 mg/kg. ATBE 50 or 25: Aqueous trunk bark extract of Markhamia tomentosa at the dose of 50 or 25 mg/kg.
Analysis of biochemical parameters
The determination of biochemical indicators such as transaminases, gamma-glutamyl-transferase, triglycerides, total protein, creatinine, total cholesterol, and total bilirubin was performed according to the kit protocols (Chronolab systems, Barcelona, Spain), revised in 2017. The analysis of superoxide dismutase, malondialdehyde, catalase, and reduced glutathione was done according to the Madoui protocols.[26]
Histological analysis
Liver and kidney samples previously kept in buffered formalin (10%) were subjected to the following histological techniques.[22] The samples were fixed, dehydrated, and impregnated. After impregnation, the samples were then embedded in the paraffin to form blocks. A 50 µm thick section of liver and kidney tissues was stained with hematoxylin-eosin and observed under a light microscope (ZEISS Axioskop, Paris, France).
Statistical analysis
Values are expressed as mean ± SEM. Statistical differences between groups were determined using analysis of variance followed by Dunnett’s test. Data were analyzed using GraphPad Prism (version 5.03). P ≤ 0.05 was considered statistically significant.
RESULTS
Qualitative phytochemical composition of the extract
The phytochemical test revealed the presence of polyphenols, tannins, flavonoids, saponins, anthocyanins, and anthraquinones in the aqueous trunk bark extract [Table 2]. Alkaloids and triterpenes were not found in the aqueous trunk bark extract of M. tomentosa.
| Compounds found | Observations |
|---|---|
| Polyphenols | + |
| Tannins | + |
| Flavonoids | + |
| Anthraquinones | + |
| Alkaloids | − |
| Triterpenes | − |
| Saponins | + |
| Anthocyanins | + |
+: Present, −: Absent
Body weight gain, liver and kidney absolute and relative weights
The results show normal growth of healthy animals with a weight gain in relation to the 1st day of treatment of 0.50 ± 3.43% on the 4th day and of 15.32 ± 2.10% on the 14th day [Table 3]. Treatment of animals with CCl4 resulted in a significant loss of body mass (−1.11 ± 2.16 and −4.74 ± 2.91%), respectively, on the 4th and 14th days, compared to the healthy control. Treatment with silymarin (25 mg /kg) prevented body weight loss until the 4th day (0.45 ± 0.99%), after which weight loss was recorded until the 14th day (−4.17 ± 1.51%). Pre-treatment with the extract (25 mg/kg) significantly improved weight gain during treatment but a significant weight loss was registered at the end of the experimental period (−2.49 ± 3.33%), compared to the healthy control group. Administered alone, the extract did not impair animal growth during the study as compared to the healthy group.
| Treatment | Body weight gain (%) | Absolute weight (g) | Relative mass (%) | ||||
|---|---|---|---|---|---|---|---|
| Day 4th | Day 8th | Day 14th | Liver | Kidney | Liver | Kidney | |
| H2O+CCl4 | −1.11±2.16# | −1.40±2.30 | −4.74±2.91# | 4.69±0.32 | 0.97±0.05 | 3.40±0.13 | 0.71±0.04 |
| H2O+Olive oil | 0.50±3.43* | 0.33±4.09 | 15.32±2.10* | 4.90±0.15 | 0.96±0.05 | 3.50±0.06 | 0.68±0.03 |
| Sily 25+CCl4 | 0.45±0.99* | −2.49±0.59 | −4.17±1.51# | 4.05±0.20# | 0.88±0.05 | 3.17±0.18 | 0.68±0.03 |
| ATBE 50+ Olive oil | 3.25±0.12* | 8.63±1.11#* | 20.99±1.18* | 5.02±0.15 | 1.01±0.02 | 3.06±0.07*## | 0.61±0.01 |
| ATBE 50+CCl4 | 4.68±0.74* | 4.05±0.31* | −2.49±3.33# | 4.14±0.05 | 0.95±0.02 | 3.02±0.02*## | 0.69±0.01 |
| ATBE 25+CCl4 | 5.11±2.43* | 1.74±2.85 | 5.91±4.54# | 4.66±0.37 | 0.90±0.06 | 3.20±0.14 | 0.62±0.00 |
Each value represents the mean±SEM. n=5 number of animals in each group. *P<0.05 compared to hepatitis control group. #P<0.05 compared to the healthy control group. Sily 25: Silymarin at a dose of 25 mg/kg. ATBE 50 or 25: Aqueous trunk bark extract of Markhamia tomentosa at the dose of 50 or 25 mg/kg, CCl4: Carbon tetrachloride.
Hepatitis control rats showed a slight reduction of the relative liver mass (3.40 ± 0.13%) compared to healthy animals (3.50 ± 0.06%). Silymarin treatment did not affect the relative liver mass of rats compared to different controls groups (3.17 ± 0.18%). A decrease in the relative mass of the liver was recorded at the end of the study in animals treated with the aqueous trunk bark extract of M. tomentosa. This reduction was significant at the dose of 50 mg/kg (3.02 ± 0.02%). A significant reduction was also observed after treatment of healthy animals with the plant extract (3.06 ± 0.07%), as compared to control groups. At the end of the treatment, no significant variation in the relative kidney weight was observed in different groups [Table 3].
Effect of the extract on enzymatic biochemical indicators of liver function
The treatment of animals with CCl4 resulted after 14 days in a significant increase in the activity of alanine aminotransferase (795.78 ± 15.78 U/L) compared to the healthy control (189.30 ± 50.42 U/L). Treatment with silymarin (25 mg/kg) significantly reduced ALT activity to 207.21 ± 31.68 U/L. Plant trunk bark extract (25 or 50 mg/kg) also reduced in a significant manner the ALT activity at 559.39 ± 91.69 U/L or 364.65 ± 39.00 U/L, respectively, compared to the hepatitis control [Table 4].
| Treatments | ALT (U/L) | AST (U/L) | γ-GT (U/L) |
|---|---|---|---|
| H2O+CCl4 | 795.78±15.78## | 487.08±57.92## | 46.16±4.32## |
| H2O+Olive oil | 189.30±50.42** | 273.11±36.76** | 17.59±3.26** |
| Sily 25+CCl4 | 207.21±31.68** | 297.35±20.89* | 23.60±2.87** |
| ATBE 50+Olive oil | 219.04±31.54** | 301.70±18.60* | 24.00±1.88** |
| ATBE 50+CCl4 | 364.65±39.00** | 353.79±41.57 | 15.42±1.48** |
| ATBE 25+CCl4 | 559.39±91.69##* | 396.47±66.01 | 21.74±4.69** |
Each value represents the mean±SEM. n=5 number of animals in each group. *P<0.05, **P<0.01, compared to the hepatitis control group, ##P<0.01 compared to the healthy control group. Sily 25: Silymarin at a dose of 25 mg/kg. ATBE 50 or 25: Aqueous trunk bark extract of Markhamia tomentosa at the dose of 50 or 25 mg/kg, CCl4: Carbon tetrachloride.
At the end of treatment, CCl4 caused a significant increase in aspartate aminotransferase activity (487.08 ± 57.92 U/L) compared to the healthy control (273.11 ± 36.76 U/L). Treatment with silymarin resulted in a significant reduction in AST activity (297.35 ± 20.89 U/L) compared to the hepatitis control [Table 4]. The extract (25 or 50 mg/kg) weakly decreased AST activity to 396.47±66.01 U/L or 353.79 ± 41.57 U/L, respectively, compared to healthy control.
A significant increase in the level of gamma-GT (46.16 ± 4.32 U/L) was recorded on the 14th day, in animals after induction of hepatitis with CCl4 as compared to the healthy control (17.59 ± 3.26 U/L). The treatment with silymarin significantly reduced the level of gamma-GT (23.60 ± 2.87 U/L); likewise, the rats treated with the plant extract (25 or 50 mg/kg) had a significantly decreased gamma-GT activity (21.74 ± 4.69 or 15.42 ± 1.48 U/L, respectively), compared to the hepatitis control [Table 4]. M. tomentosa trunk bark aqueous extract did not affect the activity of enzymatic biochemical parameters of the liver function of healthy animals (24.00 ± 1.88 U/L) as compared to healthy control.
Effect of the extract on non-enzymatic biochemical indicators of liver and renal functions
Induction of hepatitis by CCl4 injection caused 2 weeks after its administration, a significant increase in the concentration of total bilirubin (0.64 ± 0.09 mg/dL) compared to the healthy control (0.31 ± 0.05 mg/dL). Treatment of animals with silymarin significantly prevented the elevation of total bilirubin concentration (0.27 ± 0.04 mg/dL) compared to the hepatitis control [Table 5]. Administration of the extract (25 or 50 mg/kg) significantly reduced the level of total bilirubin (0.29 ± 0.12 or 0.23 ± 0.00 mg/dL, respectively) compared to the hepatitis control.
| Treatments | Total bilirubin (mg/dL) | Creatinine (mg/dL) | Total protein (mg/dL) | Triglycerides (mg/dL) | Total cholesterol (mmol/L) |
|---|---|---|---|---|---|
| H2O+CCl4 | 0.64±0.09# | 0.65±0.06 | 6.20±0.51 | 166.26±9.65 | 80.72±4.53## |
| H2O+Olive oil | 0.32±0.05* | 0.52±001 | 5.33±0.64 | 130.09±11.60 | 52.31±6.38** |
| Sily 25+CCl4 | 0.27±0.04** | 0.46±0.01** | 3.40±0.63** | 148.48±15.40 | 61.29±5.93 |
| ATBE 50+Olive oil | 0.35±0.05* | 0.47±0.01* | 9.27±0.28*## | 151.67±32.34 | 75.27±0.84# |
| ATBE 50+CCl4 | 0.23±0.00** | 0.56±0.04 | 6.68±0.12 | 116.86±14.13 | 75.37±4.66# |
| ATBE 25+CCl4 | 0.29±0.12* | 0.53±0.04 | 6.65±0.72 | 149.87±19.73 | 79.72±7.95## |
Each value represents the mean±SEM. n=5 number of animals in each group. *P<0.05, **P<0.01 compared to hepatitis control group; #P<0.05, ##P<0.01 compared to healthy control group. Sily 25: Silymarin at a dose of 25 mg/kg. ATBE 50 or 25: Aqueous trunk bark extract of Markhamia tomentosa at the dose of 50 or 25 mg/kg, CCl4: Carbon tetrachloride.
No significant increase in creatinine level was observed between the healthy control (0.52 ± 0.01 mg/dL) and hepatitis control (0.65 ± 0.06 mg/dL). Silymarin (25 mg/kg) significantly reduced creatinine levels (0.46 ± 0.01 mg/kg) compared to the hepatitis control. The extract (25 or 50 mg/kg) did not significantly influence plasma creatinine concentration (0.53 ± 0.04 or 0.56 ± 0.04 mg/dL, respectively), compared to the hepatitis control [Table 5]. In the extract control group (50 mg/kg), a significant reduction of creatinine level (0.47 ± 0.01 mg/kg) was observed, compared to the hepatitis control.
The healthy control (5.33 ± 0.64 mg/dL) and hepatitis control (6.20 ± 0.51 mg/dL) show no non-significant variation in the plasmatic level of total proteins, for in the case in rats treated with the silymarin (25 mg/kg), there is a significant reduction in the level of total proteins (3.40 ± 0.63 mg/dL) compared to hepatitis control. The rats treated with the extract (25 or 50 mg/kg) as well as the extract control group show an increase in the level of total proteins with a significant effect in the animals having received only the plant extract (9.27 ± 0.28 mg/dL) compared to the healthy and hepatitis controls.
No significant variation in triglyceride level was observed in the different groups compared to the healthy and hepatitis control.
The administration of CCl4 induced a significant increase in the total cholesterol level (80.72 ± 4.53 mmol/L) compared to the healthy control (52.31 ± 6.38 mmol/L). Treatment with the extract (25 or 50 mg/kg) and silymarin (25 mg) did not significantly reduce total cholesterol levels (79.72 ± 7.95; 75.37 ± 4.66; or 61.29 ± 5.93 mmol/L, respectively) compared to the hepatitis control. Taken alone, M. tomentosa extract induced a significant increase in cholesterol levels (75.27 ± 0.84 mmol/L) compared to that of the healthy control.
Effect of the extract on hepatic and renal oxidative stress
CCl4 induced in animals a significant increase in the level of hepatic malondialdehyde (3.13 ± 0.01 nmol/mg of protein) compared to the healthy control (2.48±0.01 nmol/ mg of protein). Treatment with silymarin (25 mg/kg) led to a significant reduction in the level of malondialdehyde (2.36 ± 0.06 nmol/mg of protein). The treatment with an aqueous extract (25 or 50 mg/kg) resulted in a significant drop in the level of malondialdehyde (2.67 ± 0.03 or 2.79 ± 0.04 nmol/mg of protein, respectively) compared to the hepatitis control [Table 6]. The extract control animals have a significantly lower level of malondialdehyde (1.65 ± 0.00 nmol/mg of protein) than the healthy control. CCl4 caused a significant increase in the level of malondialdehyde in the kidney (2.75 ± 0.02 nmol/mg of protein) compared to the healthy control (1.23 ± 0.00 nmol/mg of protein). M. tomentosa extract (25 or 50 mg/kg) significantly reduced malondialdehyde level to 1.65 ± 0.00 or 1.66 ± 0.05 nmol/ mg of protein, respectively, compared to the hepatitis control.
| Tissue | Treatment | MDA (nmol/mg of protein) |
Catalase (nmol/min/mg of protein) |
SOD (U/mg of protein) |
Glutathione (nmol/mg of protein) |
|---|---|---|---|---|---|
| Liver | H2O+CCl4 | 3.13±0.01## | 100.40±5.23# | 23.66±2.91## | 267.64±8.25## |
| H2O+Olive oil | 2.48±0.01** | 77.19±5.10* | 49.16±0.26** | 369.09±5.26** | |
| Sily25+CCl4 | 2.36±0.06** | 108.67±17.10# | 30.54±5.77# | 346.81±6.32* | |
| ATBE50+Olive oil | 1.65±0.00**## | 167.30±6.26**## | 33.37±7.79 | 390.29±29.35** | |
| ATBE50+CCl4 | 2.79±0.04**## | 155.32±12.82**## | 28.52±6.08# | 391.82±30.61** | |
| ATBE25+CCl4 | 2.67±0.03**## | 136.08±12.98# | 26.66±2.51# | 241.47±9.17# | |
| Kidney | H2O+CCl4 | 2.75±0.02## | 81.26±9.53 | 24.5±5.11# | 156.76±13.68 |
| H2O+Olive oil | 1.23±0.00** | 67.32±7.71 | 42.25±6.07* | 226.76±12.21 | |
| Sily25+CCl4 | 1.33±0.00** | 74.05±18.87 | 38.29±2.83 | 190.58±1.44 | |
| ATBE50+Olive oil | 1.63±0.03**## | 150.99±11.73**## | 36.37±2.52 | 207.94±31.60 | |
| ATBE50+CCl4 | 1.66±0.05**## | 160.13±9.51**## | 36.25±9.93 | 340.29±30.61**## | |
| ATBE25+CCl4 | 1.65±0.00**## | 141.85±16.12*## | 37.08±1.14 | 310.88±19.97**# |
Each value represents the mean±SEM. n=5 number of animals in each group. *P<0.05, **P<0.01 compared to hepatitis control group; #P<0.05, ##P<0.01 compared to healthy control group. Sily 25: Silymarin at a dose of 25 mg/kg. ATBE 50 or 25: Aqueous trunk bark extract of Markhamia tomentosa at the dose of 50 or 25 mg/kg, CCl4: Carbon tetrachloride.
The results show a significant increase in catalase activity (100.40±5.23 nmol/min/mg of protein) compared to the healthy control (77.19 ± 5.10 nmol/min/mg of protein). The extract at a dose of 25 or 50 mg/kg induced a significant rise in catalase activity (136.08 ± 12.98 or 155.32 ± 12.82 nmol/min/mg of protein) compared to the hepatitis control. The extract induced a significant elevation of hepatic catalase activity of the extract control group (167.30 ± 6.26 nmol/min/mg of protein), compared to the healthy and hepatitis control groups. In the kidney, the extract (25 or 50 mg/kg) caused a significant increase (141.85 ± 16.12 or 160.13 ± 9.51, nmol/min/mg of protein, respectively) in catalase activity compared to the hepatitis control (81.26 ± 9.53 nmol/min/mg of protein). Treatment of healthy animals with the aqueous extract of M. tomentosa result in significant rise in renal catalase activity (150.99 ± 11.73 nmol/min/mg of protein) compared to control groups.
Induction of hepatitis by CCl4 caused a significant reduction in the activity of superoxide dismutase in the liver tissue (23.66 ± 2.91 U/mg of protein) compared to the healthy control (49.16 ± 0.26 U/mg of protein). Silymarin (25 mg/kg) did not significantly prevent (30.54 ± 5.77 U/mg of protein) the reduction in superoxide dismutase activity compared to the healthy control. Similarly, the extract (25 or 50 mg/kg) did not rise significantly (26.66 ± 2.51 or 28.52 ± 6.08 U/mg of protein) the activity of superoxide dismutase compared to the healthy control [Table 6].
Hepatitis due to CCl4 caused a significant reduction in glutathione (267.64 ± 8.25 nmol/mg of protein) compared to the healthy control (369.09±5.26 nmol/mg of protein). Administration of silymarin (25 mg/kg) significantly raised glutathione levels in rats (346.81 ± 6.32 nmol/mg of protein). The extract at a dose of 50 mg/kg caused a significant increase in the glutathione level (391.82 ± 30.61 nmol/mg of protein) compared to the hepatitis control. An increase in the glutathione level is noted in the extract control group (390.29 ± 29.35 nmol/mg of protein) compared to the hepatitis control [Table 6].
Effect of extract on CCl4-induced liver inflammation
Microphotography of the liver of a healthy rat shows normal and uniformly stained hepatocytes with one or two nuclei. Cells are well-organized in travel and the portal space is clearly observable [Figure 1a]. The microphotography of the liver of the CCl4-treated rat shows a remarkable invasion of the portal space and the whole hepatic parenchyma by mononuclear inflammatory leukocyte cells. Edema characterized by dilated capillaries could be found in the parenchyma where hepatocytes present dark nuclei, indicating chromatin condensation probably as a result of a reduction in cellular activity due to inflammation [Figure 1b]. The liver section also shows cells necrosis represented by a pile of cells nuclei without a plasma membrane surrounding each. When the rats were treated with silymarin (25 mg/kg) or aqueous extract (50 or 25 mg/kg), a clear improvement of the histological parameters was observed compared to the control and mononuclear inflammatory leukocyte infiltration was also reduced [Figures 1c-f].
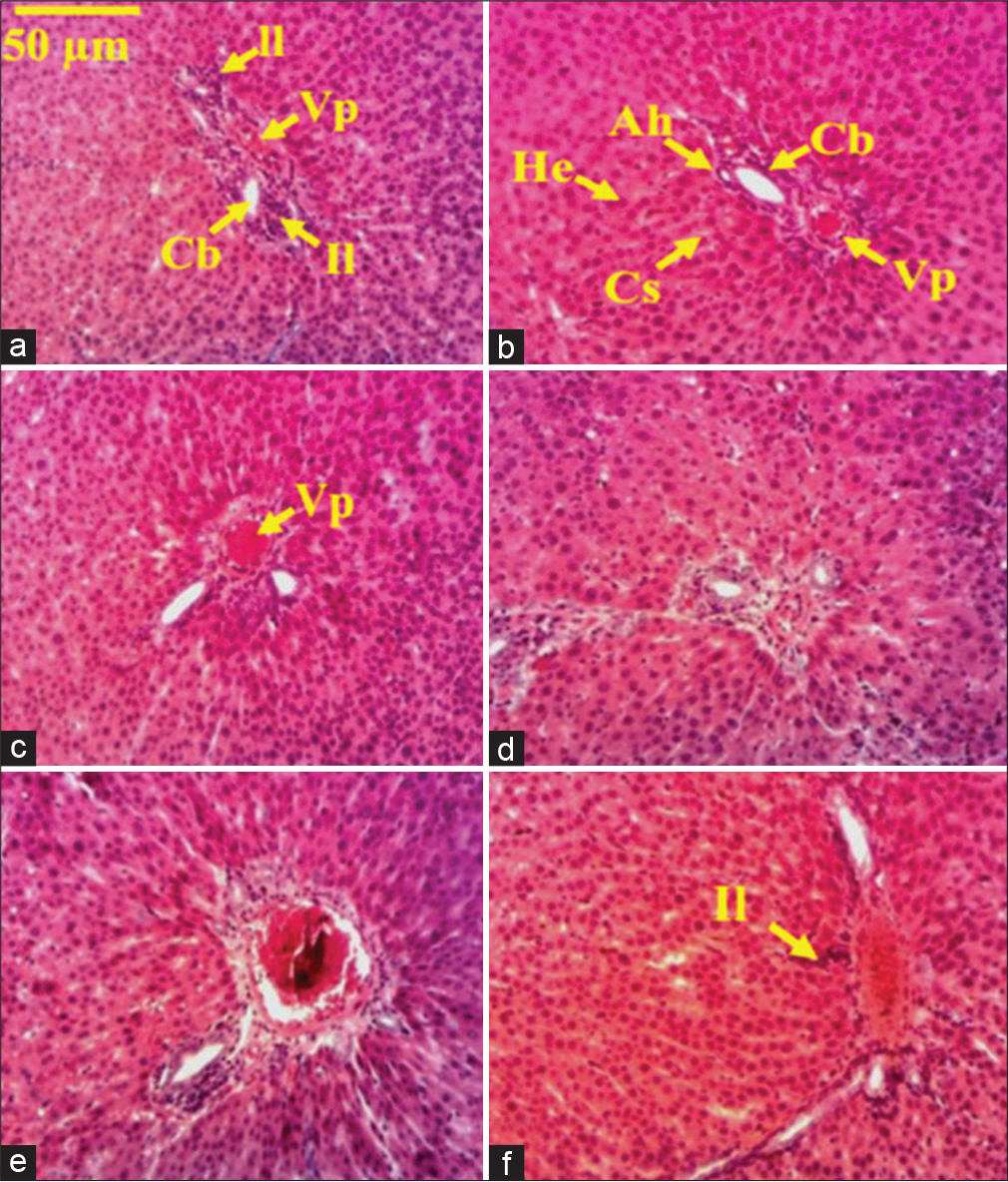
- Microphotography of the liver sections of healthy and CCl4-treated rats under Markhamia tomentosa aqueous extract treatment. Histological sections stained with hematoxylin-eosin (×100). (a) Distilled water + CCl4, (b) distilled water + olive oil, (c) silymarin (25 mg/kg) + CCl4, (d) ATBE 50 + olive oil, (e) ATBE 50 + CCl4, and (f) ATBE 25 + CCl4. VP: Portal hepatic vein, He: Hepatocytes, Cs: Sinusoidal capillary, Ah: Hepatic artery, Cb: Biliary canaliculus, Il: Leukocyte inflammation, NC: Necrosis cell, S: Steatosis. ATBE 50 or 25: Aqueous trunk bark extract of Markhamia tomentosa at the dose of 50 or 25 mg/kg, CCl4: Carbon tetrachloride.
Effect of extract on CCl4-induced kidney inflammation
Microphotography of healthy control is normal [Figure 2a]. There is an important reduction of the lumen of the proximal and distal convoluted tubes of CCl4-treated rats [Figure 2b] as compared to the healthy animal. Renal section of rats treated with silymarin (25 mg/kg) or aqueous trunk bark extract of M. tomentosa (50 or 25 mg/kg) was similar to that of healthy control [Figures 2c-f].
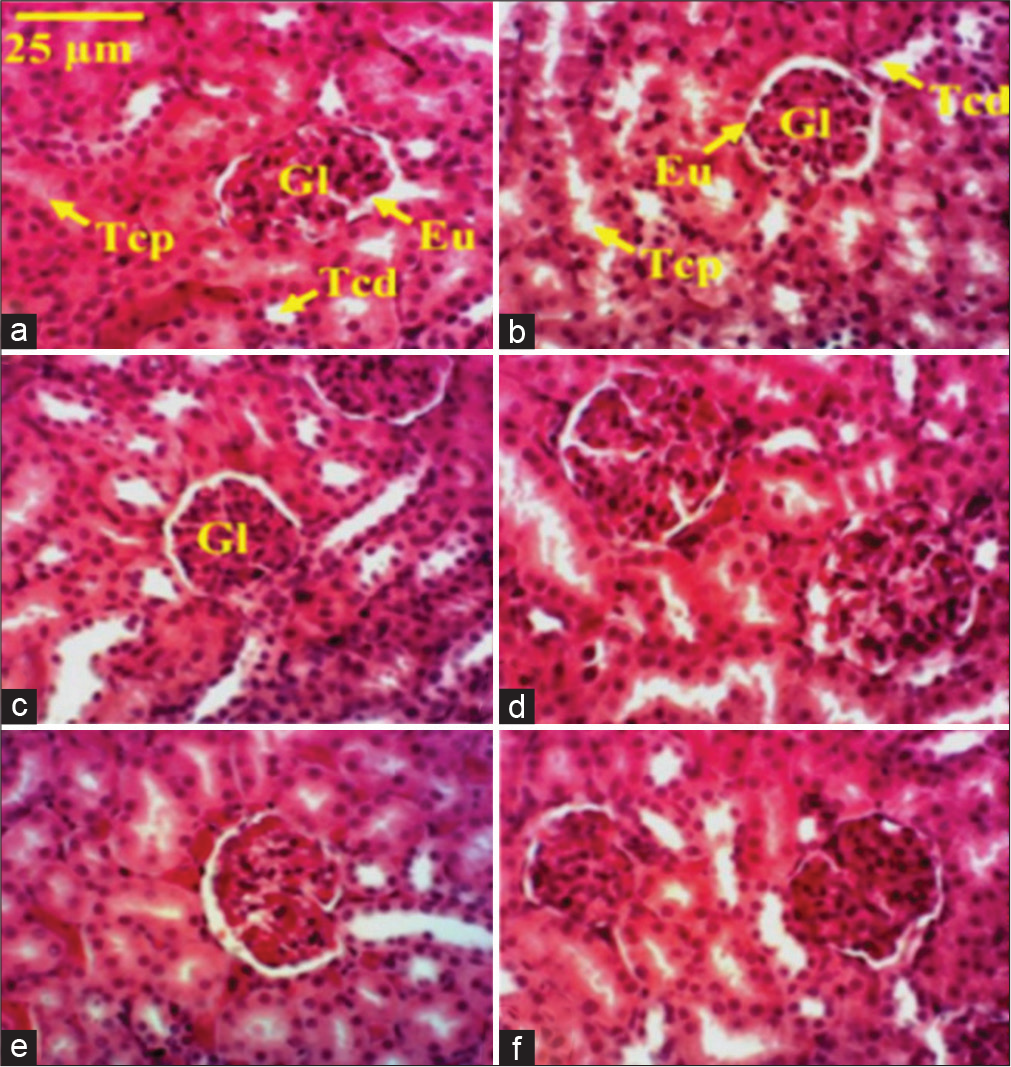
- Microphotography of the kidney sections of healthy and CCl4-treated rats under Markhamia tomentosa aqueous extract treatment. Histological sections stained with hematoxylin-eosin (×100). (a) Distilled water + CCl4, (b) distilled water + olive oil, (c) silymarin (25 mg/kg) + CCl4, (d) ATBE 50 + olive oil, (e) ATBE 50 + CCl4, and (f) ATBE 25 + CCl4. Eu: Urinary space, G: Glomerulus, Tcd: Distal involved tube, Tcp: Proximal involved tube. ATBE 50 or 25: Aqueous trunk bark extract of Markhamia tomentosa at the dose of 50 or 25 mg/kg, CCl4: Carbon tetrachloride.
DISCUSSION
This study was designed to evaluate the effects of aqueous trunk bark extract of M. tomentosa on CCl4-induced liver injury in rats since it has been claimed to be used for the management of liver diseases. Results showed that aqueous extract of M. tomentosa protects the liver against CCl4 damages. Administration of CCl4 occasioned a significant increase in liver enzyme activities, malondialdehyde levels, and induced an important leukocytes infiltration in liver tissue, which was prevented by treatment with M. tomentosa aqueous trunk bark extract.
Inflammation is a natural defense reaction of the body against any aggression, whether exogenous or endogenous. It takes place in the vascularized tissues and its ultimate goal is protection against all dangers. The liver, being a highly vascularized organ is, therefore, the privileged site of inflammatory reactions. It is a vital organ that is vulnerable to factors of liver damage, such as toxics and their metabolites, toxins, viruses, high-fat diet, and excessive alcohol consumption.[27] Liver inflammation caused by CCl4 is due to the lyse of hepatic cells by free reactive radicals from CCl4 biotransformation. P450 cytochrome metabolized CCl4 into CCl3, which, in turn, is processed in oxygenated condition to the CCl3OO.[28] These reactive species may cause harmful effects in the body by oxidizing macromolecules such as DNA, proteins, and polyunsaturated fatty acids. Hence, prolonged exposure to such reactive CCl4 metabolites leads to oxidative stress which results in the breakdown of membrane structure, disruption of cell energy, and protein synthesis processes, leading undoubtedly to cell injury.
Change in body weight is an adequate index to assess the seriousness of pathologies and to judge the normal functioning of the body. In addition, the ingestion of toxic substances causes a loss of body weight.[29] However, the interpretation of the results on the fluctuation of the body mass should not be done in an isolated way but it is necessary to take into account the variation of the mass and the relative mass of the organs. The results obtained show a significant decrease in the body mass of the hepatitis control animals while the mass and the relative mass of their liver do not show any significant difference compared to the healthy group, thus demonstrating liver edema in the hepatitis control. The rats treated with silymarin showed a significant loss of weight at the end of the treatment compared to healthy animals. The average mass of their liver is likewise significantly lower than that of the healthy control, but not their relative mass. By comparing these results with those of the hepatitis controls, it appears that silymarin inhibited edema due to CCl4. The results show that the hepatitis animals treated with the extract of M. tomentosa show a markedly improved growth compared to the hepatitis control rat, thus suggesting a beneficial effect of the extract against CCl4 hepatitis. At the end of the study, the results indicate that the relative mass of the liver of hepatitis animals treated with the extract is lower than that of healthy and hepatitis controls. This could suggest an anti-edema effect of the plant extract. However, if we take into consideration the growth of healthy rats treated with the aqueous extract and their low relative liver weight, then we could also suggest that the ability of plant components would cause a reduction of the mass of hepatic tissue; and if so, the mechanisms of this effect remain to be demonstrated.
Hepatitis caused by CCl4 is characterized by an increase in plasma levels of ALT and AST.[30,31] Transaminases (ALT and AST) are enzymes found in body cells, particularly in the liver and muscles. An increase of these enzymes in the blood is an indication of hepatic cells lysis.[30] Our results showed a significantly increased level of these enzymes when compared to the healthy group. A substantial decrease level of these enzymes was observed in hepatitis rats treated with aqueous extract. These results suggest that aqueous extract contains compounds that may protect liver cells against CCl4 damage. In general, polyphenol and flavonoid compounds could prevent cell lysis due to free radicals.[32,33] Gamma-glutamyltransferase (γGT) is a glycoprotein ubiquitous enzyme mostly found in the renal, hepatic, and pancreatic cells. High levels of γGT in the blood may be a sign of liver disease or damaged bile ducts.[34] γGT is more suitable for diagnosing cholangitis, cholecystitis, and obstructive jaundice than alkaline phosphatase, leucine aminopeptidase, and transaminases. Elevated serum value of this enzyme is also observed in hepatitis induced by CCl4.[28] Our results show significant blood elevated activity of the γGT. This finding is consistent with hepatobiliary disease induced by CCl4. Serum γGT activity was significantly reduced following treatment of rats with the aqueous trunk bark extract of M. tomentosa as well as silymarin thus proving the efficiency of the plant extract against CCl4-induced liver damage. Bilirubin is a useful indicator to evaluate the excretory function and dysfunction of hepatic bilirubin metabolism in the hepatic cell.[35,36] Hepatocytes produce bilirubin from hemoglobin and excrete it in bile or urine in a conjugate form with glucuronic acid.[35,37] Bilirubin similarly leaks into the bloodstream like transaminases when the biliary system is blocked. Administration of CCl4 causes an inflammatory reaction that alters liver cells, disorganizes the hepatic parenchyma, and increasing the plasma level of bilirubin.[35,37] Our results show a significant elevation of serum total bilirubin. Treatment of animals with aqueous trunk bark extract of M. tomentosa significantly reduced blood bilirubin level. This result is in agreement with that on γGT activity, suggesting that extract has a protective effect against CCl4-induced hepatitis. Plant flavonoid compounds are a gifted class of nutraceuticals that have been reported to be able to protect against hepatic damages.[30,38] These results revealed an improvement of enzymatic and non-enzymatic parameters thus demonstrating the efficacy of the extract of trunk bark extract of M. tomentosa to preserve normal functional liver status.
The endogen antioxidant defense system is composed of superoxide dismutase, catalase, and glutathione peroxidase, which are the main enzymes involved in the protection of the body against oxidative stress, by keeping the redox balance between the pro-oxidant and antioxidant mechanisms.[39] When the antioxidant system failed to keep pro-oxidant processes under control, this results in lipids peroxidation and alteration of DNA and protein structures.[40] MDA is the end product of lipid peroxidation widely used to quantify oxidative injury.[41] Lipid peroxidation is one of the major features of the CCl4-induced hepatitis.[41] A significant elevation of MDA level in the liver and kidney of hepatitis control rats was recorded in the present study. These results are indicative of the higher level of lipid peroxidation in these tissues, signifying the failure of endogen antioxidant defense mechanisms to prevent the formation of excessive free radicals involved in cell damages. This suggestion is consistent with the results which indicate a significant depletion in the tissues level of reduced glutathione as a consequence of the reduction of the glutathione peroxidase activity, and a significant lowering of the SOD activity as well. Glutathione peroxidase is a cytosolic enzyme that uses reduced glutathione to catalyze the reduction of hydrogen peroxide or peroxide radicals to water and oxygen or alcohols and oxygen, respectively. This enzyme has a high affinity to H2O2 compared to catalase.[42] The peroxidase and reduced glutathione are considered as major defense system when the oxidative stress is not high.[43] This study revealed that treating rats with an aqueous trunk bark extract of M. tomentosa induce a significant reduction in MDA levels, indicating that this extract reduces membrane lipid peroxidation. The results also demonstrate a substantial rise in catalase activity and a decrease in glutathione rate. Hence, it is assumed that the extract’s action may depend on the endogenous antioxidant defense system. However, after treating healthy animals with an aqueous extract of M. tomentosa, an increased amount of reduced glutathione was seen, as well as an increase in catalase and SOD activity, resulting in a decreased level of MDA in tissues. The findings of this study demonstrated the preventive efficacy of M. tomentosa aqueous extract against CCl4-induced hepatic oxidative stress.
In this study, the histological alterations of liver tissue recorded in rats subjected to CCl4 are consistent with the findings of earlier studies, which indicate a major lesion of the hepatic parenchyma like hepatocytes necrosis and inflammatory leukocyte cells infiltration in liver tissue as a result of higher oxidative stress.
It has been shown that the inflammatory reaction elicited by CCl4 in liver tissue is caused by lipid peroxidation, which increases the permeability of cell membranes and hence induces cell death.[11] Furthermore, oxidative stress causes the release of cathepsins B and then TNF-α by compromising the integrity of the lysosome membrane.[11] All these processes, according to Elgawish et al.,[11] result in an upregulation of the pro-apoptotic protein p53, resulting in an inflammatory response in hepatic tissue. Early evidence suggested that cytokines such as TNF-α, IL-1, IL-6, and IL-10 are involved in the activation and enrolment of inflammatory cells in the liver.[27,44] The histological evaluation of the liver tissue revealed a significant decrease in the infiltration of inflammatory cells. This finding is consistent with the antioxidant properties early observed, like this, reveals the inhibiting effect of M. tomentosa on CCl4-induced hepatitis. Based on the current findings, M. tomentosa aqueous extract may act similarly as silymarin either by maintaining optimal redox stability in the tissue through stimulation of antioxidant and non-enzymatic molecules or by decreasing inflammatory responses mainly through mechanisms that need to be investigated.
The results of this study are consistent with those of Temdie et al.[23] which demonstrated the protective activity of the methanol leave extract of M. tomentosa on D-galactosamine/ lipopolysaccharide and those of Ibrahim et al.[45] which also reported the prophylactic and therapeutic effects of a plant of the family Bignoniaceae against paracetamol-induced liver damage in rat. These pharmacological activities are suggested to be due to bioactive components of M. tomentosa trunk bark aqueous extract such as polyphenols, flavonoids, tannins, saponins, and anthocyanins found in the aqueous trunk bark extract. Some of these major classes of bioactive compounds have been identified in the Markhamia genus[18,45] and their strong antioxidant activities have been proved.[20,32,33]
CONCLUSION
The aqueous trunk bark extract of M. tomentosa has hepatoprotective action against CCl4-induced liver damage. Its antioxidant and anti-inflammatory properties would be responsible for this effect. Indeed, M. tomentosa extract preserves the liver’s physiological functions and architecture from the inflammatory process caused by CCl4 administration, by inhibiting lipid peroxidation, improving the endogen antioxidant defense system, and reducing inflammatory cell mobilization in the injured liver tissue. These findings support the use of M. tomentosa by communities to treat liver diseases. Nonetheless, further research is needed at this time to use this plant as an alternative treatment for liver diseases.
Acknowledgments
The authors thank those who evaluated this research work at all levels.
Authors’ contributions
All authors have participated to the realization of this work either by conceiving and designing the experiments, by conducting work on the field and data collecting, by contributing to data analysis, interpretation, and the preparation of manuscript. The final version of the article was approved by authors for publication.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- In vivo hepatoprotective effects of Rhoicissus tridentata subsp cuneifolia a traditional Zulu medicinal plant, against CCl4-induced acute liver injury in rats. South Afr J Bot. 2007;73:372-7.
- [CrossRef] [Google Scholar]
- Pharmacological importance of Clerodendrum genus: A current review. Int J Pharm Sci Res. 2017;2:22-30.
- [Google Scholar]
- Systemic inflammation mediates the effects of endotoxemia in the mechanisms of heat stroke. Biol Med. 2016;9:1000376.
- [CrossRef] [Google Scholar]
- Protective effect of Terminalia belerica Roxb, and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol. 2007;109:214-8.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular process in acute liver injury and regeneration induced by carbon tetrachloride. Life Sci. 2004;75:1539-49.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105-36.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the hepatoprotective effect of combination between hinokiflavone and glycyrrhizin against CCl4 induced toxicity in rats. Saudi Pharm J. 2018;26:496-503.
- [CrossRef] [PubMed] [Google Scholar]
- Apocynin prevented inflammation and oxidative stress in carbon tetra chloride induced hepatic dysfunction in rats. Biomed Pharmacother. 2017;92:421-8.
- [CrossRef] [PubMed] [Google Scholar]
- Rosmarinic acid ameliorates acute liver damage and fibrogenesis in carbon tetrachloride-intoxicated mice. Food Chem Toxicol. 2013;51:370-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of oxidative stress during apoptosis and necrosis caused by carbontetrachloride in rat liver. Biochim Biophys Acta. 2001;1535:186-91.
- [CrossRef] [PubMed] [Google Scholar]
- Green tea extract attenuates CCl4-induced hepatic injury in male hamsters via inhibition of lipid peroxidation and p53-mediatedapoptosis. Toxicol Rep. 2015;2:1149-56.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatoprotective activity of Chrysophyllum albidum against carbon tetrachloride induced hepatic damage in rats. Can J Pure Appl Sci. 2011;3:1597-602.
- [Google Scholar]
- Savanna Plants In: An Illustrated Guide (Illustrate). Basingstoke: Macmillan Educational Corps; 1989.
- [Google Scholar]
- Plantes Medicinales de Cote d'Ivoire. O.R.S.T.O.M. Paris: Office de la Recherche Scientifique et Technique Outre-mer; 1974.
- [Google Scholar]
- Arbres, arbustes et lianes des zones sèches d'Afrique de l'Ouest In: CIRAD, MNHN, Montpellier (2eme ed). 2000.
- [Google Scholar]
- Safety assessment of Markhamia tomentosa (benth.) K. schum. (Bignoniaceae) leaves extracts, highlight the psychostimulant effect of the methanol extract. J Exp Appl Trop Biol. 2021;1:37-47.
- [Google Scholar]
- Antiprotozoal activities of some constituents of Markhamia tomentosa (Bignoniaceae) Ann Trop Med Parasitol. 2010;104:391-8.
- [CrossRef] [PubMed] [Google Scholar]
- Analgesic and anti-inflammatory effects of extracts from the leaves of Markhamia tomentosa (benth.) K. schum. (Bignoniaceae) Pharmacologia. 2012a;3:565-73.
- [CrossRef] [Google Scholar]
- Antimicrobial and antioxidant activities of some Nigerian medicinal plants. Afr J Tradit Complement Altern Med. 2006;4:173-84.
- [CrossRef] [PubMed] [Google Scholar]
- Acute and chronic anti-inflammatory effects of the methanol leaf extract of Markhamia tomentosa (benth.) K. schum. (Bignoniaceae) J Sci Res Pharm. 2012b;1:12-8.
- [Google Scholar]
- The effects of the methanol leaf extract of Markhamia Tomentosa (benth.) K. schum. (Bignoniaceae) on arthritis induced by complete freund 's adjuvant in rats. World J Pharm Pharm Sci. 2016;5:79-92.
- [Google Scholar]
- Protective activity of Markhamia tomentosa (benth.) K. schum.(Bignoniaceae) methanol leaves extract against D-galactosamine/lipopolysaccharide-induced acute liver injury in mice. J Biosci Med. 2020;8:74-89.
- [CrossRef] [Google Scholar]
- Free radical scavenging activity, flavonoid and phenolic contents of selected Ivoirian plants. Int J Nat Appl Sci. 2007;3:425-9.
- [CrossRef] [Google Scholar]
- Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-40.
- [CrossRef] [PubMed] [Google Scholar]
- Activités biologiques des extraits de Cytisus Triflorus. Thèse de doctorat en Biochimie, Département de Sciences Biologiques, Université de Ferhat Abbas Sétif 1 d'Algérie.
- [Google Scholar]
- Acetaminophen induces liver injury and depletes glutathione in mice brain: Prevention by Moringa oleifera extract. S Afr J Bot. 2020;129:317-23.
- [CrossRef] [Google Scholar]
- Protective effect of glycyrrhizc acid against carbon tetrachloride-induced liver fibrosis in rats: Role of Integrin subunit β like 1 (ITG β l1) Slov Vet Res. 2019;56:673-9.
- [CrossRef] [Google Scholar]
- Substitution effects of a carbonated hydroxyapatite biomaterial against intoxication chloride nickel-exposed rats. Toxicol Mech Methods. 2015;25:155-65.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatoprotective effects of polymethoxyflavones against acute and chronic carbon tetrachloride intoxication. Food Chem Toxicol. 2016;91:91-9.
- [CrossRef] [PubMed] [Google Scholar]
- Les dosages sanguins liés aux maladies hépatiques. Centre Hépato-biliaire, Hôpital Universitaire Paul Brousse-12-14 avenue Paul Vaillant Couturier-F-94800 Villejuif-Fance.
- [Google Scholar]
- The protective effect of rutin and quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pac J Trop Biomed. 2017;7:647-53.
- [CrossRef] [Google Scholar]
- Carbon tetrachloride-induced hepatotoxicity in rats-protective role of aqueous leaf extracts of Coccinia grandis. Int J Pharm Tech Res. 2009;1:1612-5.
- [Google Scholar]
- Conduite à tenir devant une élévation des gamma-glutamyltransférases. 2007
- [CrossRef] [Google Scholar]
- In vitro antioxidant activity and hepatoprotective effects of Lentinula edodes against paracetamol-induced hepatotoxicity. Molecules. 2010;15:4478-89.
- [CrossRef] [PubMed] [Google Scholar]
- Carbon tetrachloride-induced hepatotoxicity in rat is reversed by treatment with riboflavin. Int Immunopharmacol. 2014;21:383-8.
- [CrossRef] [PubMed] [Google Scholar]
- Zingiber officinale roscoe prevents acetaminophen-induced acute hepatotoxicity by enhancing hepatic antioxidant status. Food Chem Toxicol. 2011;45:2267-72.
- [CrossRef] [PubMed] [Google Scholar]
- Radical scavenging activities of Lagerstroemia speciosa (L.) pers. Petal extracts and its hepato-protection in CCl4-intoxicated mice. BMC Complement Altern Med. 2017;17:55.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with Type 2 diabetes. BMC Res Notes. 2017;10:141.
- [CrossRef] [PubMed] [Google Scholar]
- Reducing oxidative stress and hepatoprotective effect of water extracts from Pu-erh tea on rats fed with high-fat diet. Food Sci Human Wellness. 2016;5:199-206.
- [CrossRef] [Google Scholar]
- Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicolology. 2003;189:113-27.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant properties of wheat bran against oxidative stress. Wheat and rice in disease prevention and health benefits, risks and mechanisms of whole grains in health promotion 2014
- [CrossRef] [Google Scholar]
- Reactive oxygen species, oxidative stress, and vascular biology in hypertension. Comprehensive Hypertension 2007
- [CrossRef] [Google Scholar]
- Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274-83.
- [CrossRef] [PubMed] [Google Scholar]
- Review of the phytochemical and pharmacological studies of the genus Markhamia. Pharmacogn Rev. 2016;10:50-9.
- [CrossRef] [PubMed] [Google Scholar]







