Translate this page into:
Quantitative assessment of residual neuromuscular blockade in post-anesthesia care unit

*Corresponding author: Lia Elsa Varghese, Department of Anaesthesiology, Bharati Vidyapeeth (deemed to be) University Medical College Hospital and Research Centre, Pune, Maharashtra, India. liaelsa.lev@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Varghese LE, Keniya VM. Quantitative assessment of residual neuromuscular blockade in post-anesthesia care unit. Adesh Univ J Med Sci Res. doi: 10.25259/AUJMSR_35_2024
Abstract
Objectives:
Residual neuromuscular blockade (RNMB) is a critical factor contributing to post-operative respiratory complications, even when traditional extubation criteria are met. This study aimed to assess the incidence of residual neuromuscular blockade (RNMB) using train of four ratios and identify associated risk factors in post-surgical patients.
Material and Methods:
After ethical permission and written informed consent, this prospective observational study was undertaken in tertiary care hospital in 80 American Society of Anesthesiologists (ASA) Grade I and II patients aged 18–60 years undergoing surgery of duration more than 2 h. Patients with renal or liver disease, neuromuscular disorders, or hemodynamic instability were excluded. During the operation under general anesthesia, data on total drug usage, hemodynamic parameters, blood loss, urine output, and temperature were all recorded. Following extubation, RNMB was evaluated serially using train-of-four (TOF) watch acceleromyography, with the first measurement recorded at extubation (T0), at 5-min until the TOF ratio >0.9 was obtained. Statistical analysis was performed with the Statistical Package for the Social Sciences, with P < 0.05 considered significant.
Results:
The incidence of RNMB was 28.8%. Patients with RNMB were mainly female (69.6%), older (average age 42.61 years), and had a higher body mass index (BMI) (26.00 kg/m2) compared to those without RNMB. Prolonged surgery duration, increased muscle relaxant dosage, and intraoperative use of labetalol and tranexamic acid were significantly associated with RNMB. No significant association was observed with ASA grades, hypertension, diabetes, or type of surgery.
Conclusion:
Residual neuromuscular blockade (RNMB) remains a significant post-operative risk, particularly in females, older patients, and those with higher BMI. Quantitative neuromuscular monitoring is crucial for complete recovery and reducing complications. While clinical extubation criteria work well for ASA I and II patients, intraoperative TOF monitoring is recommended for those with higher BMI, longer surgeries, or significant blood loss. This study supports the reliability of clinical criteria in low-risk groups and calls for further research on higher-risk populations, such as ASA III and IV patients.
Keywords
Neuromuscular blocking agents
Residual neuromuscular blockade
Train-of-four
INTRODUCTION
Neuromuscular blocking agents (NMBAs) are indispensable for aiding endotracheal intubation and achieving muscle relaxation throughout the surgical procedure. However, the management of neuromuscular recovery postoperatively remains a significant challenge, particularly in preventing postoperative residual curarization (PORC). PORC, defined as residual neuromuscular blockade after NMBA use, is associated with complications such as aspiration, airway obstruction, and impaired ventilatory response. Despite traditional extubation criteria – such as assessments of vital capacity, maximum inspiratory pressure, and clinical signs such as fully awake patient, spontaneous breathing pattern sustained head lift for 10 seconds, and assessment of muscle tone and strength – residual paralysis often goes undetected due to their limited sensitivity and reliance on patient cooperation.[1,2]
A more precise technique for evaluating neuromuscular recovery is quantitative neuromuscular monitoring, such as train-of-four (TOF) ratio measures. For safe extubation, a TOF ratio (TOFR) of 0.9 or higher is deemed essential. However, reliance on clinical tests, such as head-lift and tongue depression, has been shown to inadequately detect residual blockade, with sensitivity as low as 11–14%. Even with the use of intermediate-acting NMBAs and regular clinical evaluations, recent research highlights the ongoing danger of residual paralysis. Debaene et al. discovered that patients who received intermediate-acting NMBAs such as vecuronium, rocuronium, and atracurium without reversal agents had a 45% frequency of residual blockage (TOFR <0.9).[3]
Similarly, Baillard et al., found that 42% of patients had residual neuromuscular blockade (RNMB) (TOFR <0.7) when they arrived in the post-anesthesia care unit (PACU) and that inadequate recovery led to adverse consequences like reintubation.[4]
These findings highlight the need for standardized use of quantitative neuromuscular monitoring to ensure complete recovery and improve postoperative outcomes. Since our institution still uses traditional clinical criteria for extubation, therefore, our study aims to assess the incidence of residual neuromuscular blockade (RNMB) in PACU and pinpoint contributing factors to guide improvements in patient safety practices.
MATERIAL AND METHODS
After ethical committee permission, this prospective observational study was conducted at a tertiary care hospital from June 2022 to January 2024 in a total of 80 patients, based on the inclusion criteria and after providing written informed consent. Patients included in the study were American Society of Anesthesiologists (ASA) Grade I and II, aged between 18 and 60 years, and scheduled for surgeries requiring general anesthesia lasting more than 2 h with neuromuscular blockade. Pre-operative assessments were conducted, including detailed medical histories, clinical examination, and baseline investigations. Patients were excluded if they had renal or liver disease, neuromuscular disorders, hemodynamic instability, or if they refused consent.
The sample size calculation was performed based on an estimated incidence of PORC (TOF <0.9) of 0.3, aiming for a power of 80% and a type 1 error rate of 5%, referencing previous studies on PORC.[5]
A standardized anesthesia protocol was followed throughout the study. Pre-medication was administered intravenously with glycopyrrolate (0.1 mg/kg). Pre-induction medications included midazolam (70–80 mcg/kg) and fentanyl (0.5–2 mcg/kg), followed by induction with propofol. Neuromuscular blockade was achieved using atracurium. Anesthesia was maintained using sevoflurane, with additional adjuvants (dexmedetomidine, clonidine, or xylocard) and analgesics (paracetamol or tramadol) as necessary. Intraoperative monitoring included continuous recording of hemodynamic parameters (heart rate, blood pressure, oxygen saturation), urine output, blood loss, core body temperature, and other intraoperative events.
After surgery, once spontaneous muscle activity returned, neuromuscular blockade was reversed using Neostigmine (0.04 mg/kg IV) and Glycopyrrolate (0.1 mg/kg IV). Extubation was performed only after confirming adequate muscle strength and respiratory function based on standard extubation criteria.
In the PACU, residual neuromuscular blockade was assessed using the TOFR, measured by TOF-Watch acceleromyography. Ulnar nerve stimulation with four pulses of 0.2 ms duration at a frequency of 2 Hz and supra-maximal stimulation of 50 mA was applied. The TOFR was recorded every 5 min until a TOFR of ≥0.9 was achieved, and the time to reach this threshold was documented.
Data collection included demographic details [age, sex, body mass index (BMI)] comorbidity status of the patient and medications, ASA grade, intraoperative anesthetic drugs, type of surgery, intra-operative events, hemodynamic parameters (blood loss, urine output, hypothermia), TOF values, and time to TOF ≥0.9.
All data were analyzed using Statistical Package for the Social Sciences (version 24.0), with categorical variables compared using the Chi-square test or Fisher’s exact test, and continuous variables analyzed with the independent t-test, normal distribution. A P < 0.05 was considered statistically significant. Ensuring results were presented in both tabular and graphical formats for enhanced clarity.
RESULTS
Incidence of residual neuromuscular blockade (RNMB)
Out of 80 cases studied, 23 cases (28.8%) had residual neuromuscular blockade (RNMB) and 57 cases (71.3%) did not have RNMB in the study group.
Time to achieve TOF 0.9 among the cases with RNMB
Out of 23 cases with RNMB, the majority of cases, i.e., 12 cases (52.2%) reached TOF of 0.9 at 5 min and 9 cases (39.1%) required 10 min to reach. TOF 0.9 and 2 cases (8.7%) took 15 min to reach TOF 0.9 in the study group [Figure 1].
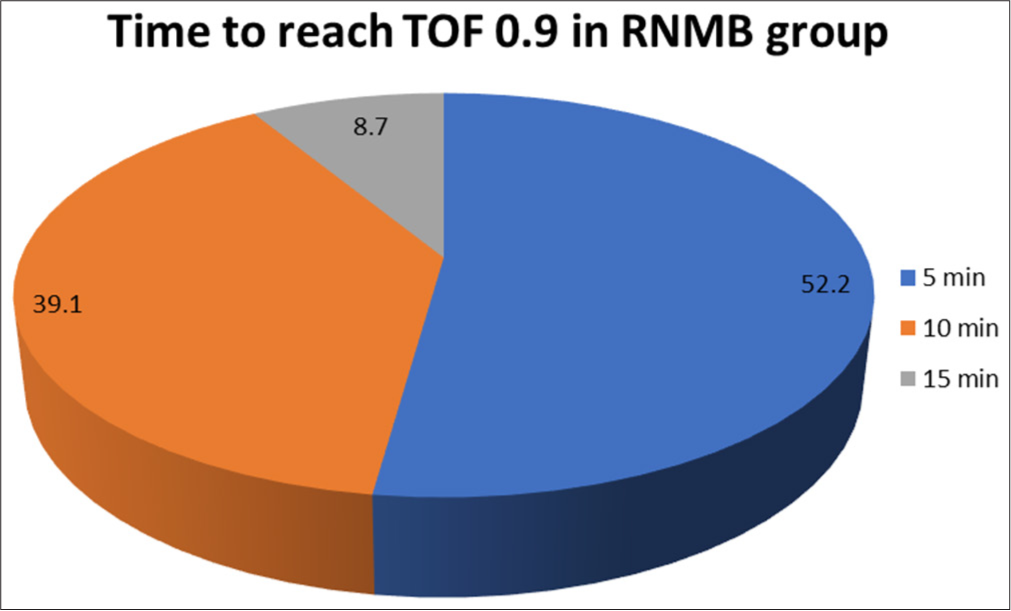
- Distribution of time to achieve train-of-four 0.9 among the cases with RNMB. TOF: Train of Four, RNMB: Residual Neuromuscular Blockade.
Distribution of patient characteristics
In the study, 23 patients exhibited residual neuromuscular blockade (RNMB), while 57 did not. Of the RNMB group, 30.4% were male, and 69.6% were female, with a significantly higher incidence of RNMB in females compared to males (P < 0.05). The average age of the RNMB group was 42.61 ± 15.29 years, significantly higher than the no-RNMB group, which had a mean age of 35.23 ± 11.84 years (P < 0.05). The RNMB group also had a significantly higher mean BMI of 26.00 ± 5.75 kg/m2, compared to 23.02 ± 3.77 kg/m2 in the no-RNMB group (P < 0.05).
Regarding ASA grading, 65.2% of RNMB cases were grade 1, and 34.8% were grade II. In contrast, 77.2% of no-RNMB cases were grade 1, and 22.8% were grade II. The incidence of RNMB did not significantly differ across ASA grades (P > 0.05). Hypertension was found in 34.8% and diabetes mellitus in 8.7% of the RNMB group, with no notable difference in the occurrence of RNMB between individuals with or without these conditions (P > 0.05). In addition, surgery type (general, gynecology, urology, and orthopedic) did not influence RNMB incidence (P > 0.05) [Table 1].
| All (n=80) | RNMB (n=23) | No-RNMB (n=57) | P-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 39 (48.7) | 7 (30.4) | 32 (56.1) | 0.049* |
| Female | 41 (51.3) | 16 (69.6) | 25 (43.9) | |
| Age (years) | 37.35±13.26 | 42.61±15.29 | 35.23±11.84 | 0.022* |
| BMI (kg/m2) | 23.87±4.59 | 26.00±5.75 | 23.02±3.77 | 0.012* |
| ASA grades | ||||
| Grade 1 | 59 (73.8) | 15 (65.2) | 44 (77.2) | 0.271NS |
| Grade 2 | 21 (26.2) | 8 (34.8) | 13 (22.8) | |
| Comorbidity | ||||
| Hypertension | 17 (22.1) | 8 (34.8) | 9 (15.8) | 0.075NS |
| Diabetes mellitus | 7 (9.1) | 2 (8.7) | 5 (8.8) | 0.999NS |
| Medication | ||||
| Anti-hypertensive | 17 (21.3) | 8 (34.8) | 9 (15.8) | 0.060NS |
| Anti-diabetic | 7 (8.8) | 2 (8.7) | 5 (8.8) | 0.999NS |
| Surgery | ||||
| General surgery | 61 (76.3) | 15 (65.2) | 46 (80.7) | 0.218NS |
| Gynecology | 2 (2.5) | 0 | 2 (3.5) | |
| Urology | 3 (3.8) | 1 (4.3) | 2 (3.5) | |
| Orthopedic | 14 (17.5) | 7 (30.4) | 7 (12.3) |
P-value for comparing n (%) by Chi-square test. P-value for comparing means by independent sample t-test. P<0.05 is considered to be statistically significant. *P<0.05, NS: Statistically non-significant, BMI: Body mass index, ASA: American Society of Anesthesiologists, RNMB: Residual Neuromuscular Blockade. Figures in the bracket indicate the percentage of cases in each group.
Intra-operative drugs
The incidence of use of intra-operative drugs such as Dexmedetomidine, Ephedrine, Dexamethasone, and Hydrocortisone did not differ significantly between a group of cases with RNMB and a group of cases with no-RNMB (P > 0.05 for all). The incidence of RNMB in cases where intra-operative drugs such as tranexamic acid (TXA) and labetalol were administered is substantially higher in the RNMB group compared to the no-RNMB group (P < 0.05 for both) [Table 2].
| Intra-operative drug given | All (n=80) | RNMB (n=23) | No-RNMB (n=57) | P-value |
|---|---|---|---|---|
| Epidural | 1 (1.3) | 0 | 1 (1.8) | 0.999NS |
| Dexmedetomidine | 44 (55.0) | 15 (65.2) | 29 (50.9) | 0.243NS |
| Ephidrine | 3 (3.8) | 1 (4.3) | 2 (3.5) | 0.999NS |
| Dexamethazone | 28 (35.0) | 11 (47.8) | 17 (29.8) | 0.127NS |
| Hydrocortisone | 8 (10.0) | 3 (13.0) | 5 (8.8) | 0.683NS |
| Tranexamic acid | 5 (6.3) | 4 (17.4) | 1 (1.8) | 0.022* |
| Labetlol | 8 (10.0) | 7 (30.4) | 1 (1.8) | 0.001*** |
P-value by Chi-Square test. P<0.05 is considered to be statistically significant. ***P<0.001, NS: Statistically non- significant. *P<0.05(Statistically Significant). RNMB: Residual Neuromuscular Blockade. Figures in the bracket indicate the percentage of cases in each group.
Mean blood loss, urine output
The mean blood loss in the RNMB group was significantly higher at 257.61 ± 143.89 mL compared to 155.10 ± 88.45 mL in the no-RNMB group (P < 0.05). Similarly, the RNMB group had a significantly higher mean urine output of 452.17 ± 140.19 ml compared to 377.78 ± 152.24 ml in the noRNMB group (P < 0.05). No cases in either group exhibited hypothermia [Table 3].
| Variable | All (n=80) | RNMB (n=23) | No-RNMB (n=57) | P-value |
|---|---|---|---|---|
| Blood loss (mL) | 186.53±117.53 | 257.61±143.89 | 155.10±88.45 | 0.001*** |
| Urine output (mL) | 400.00±151.74 | 452.17±140.19 | 377.78±152.24 | 0.048* |
| Hypothermia | 0 | 0 | 0 | -- |
P-value by independent sample t-test. P<0.05 is considered to be statistically significant. *P<0.05, ***P<0.001. ±: The symbol indicates one standard deviation (SD) added to or subtracted from the mean.
Inter-group comparison of outcome measures
The mean dose of sevoflurane used in the RNMB group (1.92 ± 0.14) and the no-RNMB group (1.91 ± 0.12) was similar, with no significant difference (P > 0.05). However, the total dosage of muscle relaxants was significantly higher in the RNMB group (71.41 ± 11.75) compared to the noRNMB group (57.68 ± 10.08) (P < 0.05). The duration of muscle relaxant administration before extubation did not significantly differ between the groups (P > 0.05). In addition, the RNMB group had a significantly longer surgery duration (156.52 ± 19.09 min) compared to the no-RNMB group (134.46 ± 15.39 min) (P < 0.05) [Table 4].
| Variable | All (n=80) | RNMB (n=23) | No-RNMB (n=57) | P-value |
|---|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | ||
| Average dose of Sevoflurane | 1.91 ± 0.12 | 1.92 ± 0.14 | 1.91 ± 0.12 | 0.888NS |
| Total dosage of muscle relaxant given (mg) | 61.63 ± 12.23 | 71.41 ± 11.75 | 57.68 ± 10.08 | 0.001*** |
| Duration of muscle relaxant given prior extubation (min) | 34.18 ± 6.27 | 34.35 ± 5.50 | 34.11 ± 6.61 | 0.903NS |
| Duration of surgery (min) | 140.89 ± 19.28 | 156.52 ± 19.09 | 134.46 ± 15.39 | 0.001*** |
P-value by independent sample t-test. P<0.05 is considered to be statistically significant. ***P<0.001, NS: Statistically non- significant, ±: The symbol indicates one standard deviation (SD) added to or subtracted from the mean.
DISCUSSION
Residual neuromuscular blockade (RNMB) has been demonstrated to be linked to considerable morbidity and delays in recovery room discharge. RNMB is frequently observed in the early post-operative phase and may persist after admission to the PACU.Even mild residual paresis, characterized by a TOFR between 0.7 and 0.9, can lead to pharyngeal and esophageal dysfunction, upper airway obstruction, reduced hypoxic ventilatory response, and notable patient discomfort. Therefore, a TOFR of 0.9 or higher is recommended as the minimum acceptable threshold for recovery.[1,2]
Clinical tests, such as coughing, eye-opening, and tongue protrusion, are often used to assess residual paralysis. Head lift, leg lift, or firm hand grasp for more than 5 s is considered reliable markers of recovery. However, these tests require patients to be awake, cooperative, and free from the residual effects of anesthesia. These conditions are not always achievable in the PACU, limiting the effectiveness of clinical tests in assessing RNMB.[3]
This prospective study involved 80 patients who underwent general anesthesia for over 2 h with NMBAs and were extubated postoperatively. RNMB was assessed using a TOFR with a TOF-Watch acceleromyography. Among these patients 28.8% were found to have RNMB. Compared to Viby-Mogensen and Jørgensen’s 1979 study, which reported a higher incidence of RNMB (42%) using clinical criteria, our findings emphasize the critical role of neuromuscular monitoring. This study also underscores the inadequacy of neostigmine in reversing RNMB in certain patients and highlights the importance of nerve stimulators for effective muscle relaxant management.[1]
Studies utilizing the TOFR <0.9 criterion show RNMB incidence rates varying from 13% to 88%, likely due to differences in research methods, such as whether intraoperative muscle biopsies were used, the types of neuromuscular monitoring equipment employed, and the reversal agent protocols followed. The choice between allowing spontaneous recovery or administering reversal agents also affects the reported RNMB rates. The incidence observed in our study aligns with this range, highlighting how our findings are consistent with the variability seen in other research.[1-5]
In our study, 28.8% of the patients experienced RNMB, with a significantly higher incidence among females (69.6%) compared to males (30.4%). Patients with RNMB were older (mean age 42.61 years vs. 35.23 years) and had a higher BMI (26.00 kg/m2 vs. 23.02 kg/m2). These findings suggest that older age, female gender, and higher BMI are risk factors for RNMB after tracheal extubation. Consistent with Murphy et al., elderly patients (70–90 years) showed a higher incidence of RNMB (57.7% vs. 30% in younger patients), linked to respiratory complications, longer PACU stays, and extended hospitalizations. This emphasizes the need for improved perioperative monitoring.[6]
Similar to our findings, decreased muscle mass, diminished organ function, and impaired thermoregulation in elderly individuals may prolong the effects of medications, including NMBAs. In addition, older individuals may have a reduced ability for anticholinesterases to reverse neuromuscular function. Women generally have less muscle mass and more adipose tissue than men, leading to a decreased volume of distribution and increased plasma concentration of muscle relaxants.[7] The same reasoning can be applied to patients with increased BMI, further predisposing them to postoperative RNMB.
Elevated BMI also presents challenges in dosing neuromuscular blocking drugs, affecting optimal muscle relaxation levels. Our study found no significant difference in RNMB incidence between ASA I and II patients, consistent with findings by Saager et al. Their multicenter study reported that 64.7% of patients experienced RNMB, with higher BMI and ASA III status increasing the likelihood of residual blockade. By excluding ASA III patients, we aimed to validate our standard care protocol for RNMB detection and reversal.[8]
In line with our findings, Esteves et al.’s study (INSPIRE 2) also found no significant correlation between RNMB and ASA grades. This suggests that clinical criteria may be sufficient to identify RNMB in ASA I and II patients when quantitative neuromuscular monitoring is unavailable. In addition, an algorithm developed by Unterbuchner et al. demonstrated the potential to prevent severe RNMB in the PACU using qualitative neuromuscular monitoring in patients with lower ASA grades.[9,10]
Our results showed that 34.8% of patients with RNMB had hypertension, compared to 15.8% of patients without RNMB. Despite this apparent difference, statistical analysis revealed no significant correlation between hypertension and RNMB (P > 0.05). Similarly, diabetes mellitus was present in 8.7% of patients with RNMB and 8.8% of those without RNMB, with no significant difference in incidence (P > 0.05). These findings align with the RECITE-US study by Saager et al., which also could not find any correlation between RNMB and comorbid conditions.[8]
The type of surgery performed also showed no significant association with RNMB in our study. Among patients with RNMB, 65.2% underwent general surgery, 4.3% underwent urological surgery, and 30.4% underwent orthopedic surgery. Similarly, 80.7% of patients without RNMB underwent general surgery, with smaller percentages undergoing gynecological, urological, and orthopedic procedures. Statistical analysis revealed no significant differences in RNMB incidence based on surgical type (P > 0.05).
The study found a substantially higher incidence of RNMB among patients who received intraoperative medications such as labetalol and TXA compared to those who did not (P < 0.05). Although labetalol is commonly used, its direct effect on neuromuscular transmission is unclear, and limited research exists on its impact on RNMB in the PACU. Similarly, while TXA is not known to cause RNMB, our findings warrant further research to draw definitive conclusions due to the study’s small sample size. Other intraoperative medications, such as epidurals, dexmedetomidine, ephedrine, dexamethasone, and hydrocortisone, did not contribute to RNMB in our study. However, the small sample size limits the reliability of these findings [Table 2].
Blood loss and urine output were substantially higher in the RNMB group compared to the no-RNMB group. The RNMB group experienced greater blood loss (257.61 ± 143.89 mL vs. 155.10 ± 88.45 mL), potentially affecting the pharmacokinetics of NMBAs and prolonging their effects. Urine output was also marginally higher in the RNMB group (P = 0.048). Effective blood loss and fluid management are critical to minimizing RNMB risk.
In this study, no cases of hypothermia were observed due to preventive measures during surgery. However, hypothermia has been shown to prolong neuromuscular blockade, emphasizing its importance in preventing RNMB. These findings underscore the need for comprehensive intraoperative and post-operative management to mitigate RNMB risk.[11]
Research by Reid et al.demonstrated that volatile agents such as sevoflurane and isoflurane can impede the antagonistic effects of neuromuscular relaxants like vecuronium. Sevoflurane anesthesia potentiates rocuronium’s effects, delaying adequate reversal of rocuronium-induced neuromuscular blockade. In our study, discontinuing sevoflurane before administering reversal agents likely contributed to the lower incidence of RNMB, highlighting the importance of proper timing in anesthetic administration.[12]
Our findings also showed that the risk of RNMB increased with the number of additional NMBD doses, consistent with Naguib et al.’s observation that high doses and short NMBD-PACU durations elevate RNMB risk. Adhering to our protocol, all patients were extubated at least 30 min after the last NMBA dose. The time from the final NMBA dose to PACU transfer showed no significant correlation with RNMB, reflecting the effectiveness of our extubation guidelines.[13]
A significant distinction in surgical duration was observed between patients with and without RNMB. The mean surgical duration in the RNMB group was 156.52 ± 19.09 min, compared to 134.46 ± 15.39 min in the no-RNMB group. Longer surgeries increase exposure to NMBAs, raising the likelihood of incomplete neuromuscular recovery. This highlights the need for heightened awareness and proactive management of NMBAs during prolonged surgeries to improve postoperative outcomes and ensure patient safety.[14]
Despite these findings, our study has several limitations. Patients with an ASA score greater than II and those requiring post-operative critical care unit admission were excluded. Future studies should include higher-risk populations (ASA III and IV). In addition, the small sample size and observational design limit the ability to establish causality. Further research with larger cohorts and diverse patient populations is essential to confirm these findings and refine perioperative neuromuscular management protocols.
CONCLUSION
For ASA I and II patients, the utilization of clinical criteria for safe extubation is considered appropriate; however, diligent observation in the PACU for 15–20 min is recommended to mitigate potential adverse outcomes. In cases involving patients with a high BMI, extended surgical duration, or anticipated increased blood loss, intraoperative monitoring of TOF is deemed safe and advisable. Future research endeavors should focus on extending this validation to higher-risk patient populations (ASA III and IV) to assess the efficacy of traditional clinical extubation criteria in these cohorts.
Ethical approval:
The research/study was approved by the Institutional Review Board at Bharati Vidyapeeth (Deemed to be University) Medical College Institutional Ethics Committee, number BVDUMC/IEC/88, dated 12th August 2022.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Residual curarization in the recovery room. Anesthesiology. 1979;50:539-41.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of residual curarization using low-current stimulation. Can J Anaesth. 1991;38:164-8.
- [CrossRef] [PubMed] [Google Scholar]
- Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology. 2003;98:1042-8.
- [CrossRef] [PubMed] [Google Scholar]
- Residual curarization in the recovery room after vecuronium. Br J Anaesth. 2000;84:394-5.
- [CrossRef] [PubMed] [Google Scholar]
- Postoperative residual curarization: Clinical observation in the post-anesthesia care unit. Chang Gung Med J. 2008;31:364-8.
- [Google Scholar]
- Residual neuromuscular block in the elderly: Incidence and clinical implications. Anesthesiology. 2015;123:1322-6.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and associated factors of residual neuromuscular block among patients underwent general anaesthesia at university of Gondar hospital, a cross-sectional study. J Anesth Crit Care Open Access. 2017;7:e00284.
- [CrossRef] [Google Scholar]
- Incidence, risk factors, and consequences of residual neuromuscular block in the United States: The prospective, observational, multicenter RECITE-US study. J Clin Anesth. 2019;55:33-41.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of postoperative residual neuromuscular blockade-a multicenter, observational study in Portugal (INSPIRE 2) Porto Biomed J. 2023;8:e225.
- [CrossRef] [Google Scholar]
- Development of an algorithm using clinical tests to avoid post-operative residual neuromuscular block. BMC Anesthesiol. 2017;17:101.
- [CrossRef] [PubMed] [Google Scholar]
- Preventive strategies of residual neuromuscular blockade in resource-limited settings: Systematic review and guideline. Int J Surg Open. 2020;26:73-80.
- [CrossRef] [Google Scholar]
- Neostigmine antagonism of rocuronium block during anesthesia with sevoflurane, isoflurane or propofol. Can J Anaesth. 2001;48:351-5.
- [CrossRef] [PubMed] [Google Scholar]
- Neuromuscular monitoring and postoperative residual curarisation: A meta-analysis. Br J Anaesth. 2007;98:302-16.
- [CrossRef] [PubMed] [Google Scholar]
- Safe tracheal extubation after general anaesthesia. BJA Educ. 2021;21:446-54.
- [CrossRef] [PubMed] [Google Scholar]







