Translate this page into:
Prevalence of basal-like phenotype in triple-negative invasive breast carcinomas and a review of the literature

*Corresponding author: Emmanuel Kunle Abudu, Department of Histopathology, University of Uyo Teaching Hospital, Uyo, Akwa Ibom State, Nigeria. ekabudu@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Obioha K, Kudamnya IJ, Akpanudo EI, Abudu EK, Abudu OO, Banjo AF. Prevalence of basal-like phenotype in triple-negative invasive breast carcinomas and a review of the literature. Adesh Univ J Med Sci Res 2023;5:60-8. doi: 10.25259/AUJMSR_18_2023
Abstract
Objectives:
The study is aimed at analyzing the clinico-pathological features and immunohistochemical expression of basal-like phenotype in triple-negative breast carcinomas (TNBC) as well as stratifying of invasive breast carcinomas into main molecular subtypes and correlating the Basal-like breast carcinoma subtype with the size of tumor, grade and age of patient in the University of Uyo Teaching Hospital, Uyo, Akwa Ibom State, Nigeria.
Material and Methods:
This study was a descriptive cross-sectional hospital-based, that was carried out in the Histopathology Department of the University of Uyo Teaching Hospital, Uyo, Akwa Ibom State, Nigeria, using paraffin-embedded tissue blocks of invasive carcinomas of breast received between July 01, 2016, and August 31, 2018, subject to re-histological assessment and IHC staining for various breast carcinoma markers, including estrogen receptor (ER), progesterone receptor (PR), human epidermal receptor-2 (HER-2), Ki67, epidermal growth factor receptor (EGFR), and CK5/6. The age of patients and tumor size was accessed from the records of the department. The data were analyzed by descriptive and inferential statistics using the Statistical Package for the Social Sciences version 16.
Results:
A total of 79 histologically confirmed invasive breast carcinomas in females were analyzed. The age range of the patients was 24–76 years, with a mean age of 43.96 ± 9 years. The majority of the invasive carcinomas were of no special type (91.1%). ER, PR, and HER-2 were positive in 53.2%, 32.9%, and 12.7% of cases, respectively. Phenotypic classification based on immunohistochemistry showed that the Luminal A subtype was the predominant subtype, accounting for 45.3% of cases, while Luminal B, HER-2 enriched, basal-like, and unclassified were 13.3%, 5.3%, 17.3%, and 18.7%, respectively. Triple-negative breast carcinomas (TNBCs) accounted for 36.0% of the cases. The frequency of basal-like breast carcinoma in TNBCs was 48.1%.
Conclusion:
Our study confirmed that the Luminal A subtype of breast carcinomas was the commonest, and there was an appreciable number of the basal-like breast carcinoma subtype in Uyo, with a frequency of 48.1% among the TNBCs.
Keywords
Breast carcinoma
Basal-like subtype
Triple-negative breast carcinomas
INTRODUCTION
Breast carcinomas are a highly heterogeneous group of cancers with a wide range of differences in its morphology, molecular genetics, biology, and clinical outcomes.[1,2] It is the most frequently diagnosed cancer among women worldwide with associated dismal outcomes.[3] The classification of breast carcinoma histologically is of the essence in prognostication and predicting response to therapy with proven limitations.[4,5] These limitations are, however overcome to some extent by the introduction of immunohistochemical (IHC) classification, which involves microscopic localization of specific antigens or proteins in tissues by staining with labeled antibodies.[6,7] The expression of antigens, for example, hormone receptors (estrogen receptor [ER], progesterone receptor [PR]), and human epidermal receptor-2 (HER-2) assign individuals to groups that can be treated with specific drugs.[6,7]
The differential staining of breast cancer for ER, PR, and HER-2 in immunohistochemistry allows pathologists to classify breast cancer into distinct subtypes which include; hormone-receptor-positive (ER+/PR+/HER-2−, ER+/PR+/HER-2+), triple-negative (ER−/PR−/HER-2−), and HER-2 positive breast cancers (ER−/PR−/HER-2+).[1,7,8]
Triple-negative breast carcinoma (TNBC) on its own is a heterogeneous disease and represents 10–17% of all breast cancers.[9,10] It is a highly aggressive carcinoma that lacks targeted therapy and is characterized by poor prognosis, high risk of distant recurrence, and death as well as a tendency to present as interval cancers due to their high proliferation rates.[11]
With the recent advances in research, breast carcinomas are now classified using gene expression profiling into four to five molecular subtypes, including Luminal (A and B), HER-2, basal-like, and normal breast subtypes that have different biology and clinical outcomes.[2,11]
Although TNBC is not one of the molecular subtypes initially identified by gene expression profiling, majority of the TNBCs fall into the basal-like subtype when subjected to molecular studies and can be further stratified into basal-like, immunomodulatory, mesenchymal, mesenchymal stem cell-like, and luminal subtype expressing androgen receptor, all of which differ in racial distribution, clinicopathologic features and therapeutic requirements, hence the need to identify such subtypes.[12]
Basal-like breast carcinoma derived its name from its propensity to express genes of normal basal myoepithelial cells of the breast and represents 10–25% of all breast cancers (depending on the population) and makes up about 50–75% of the triple-negative subtype.[13,14] The basal-like TNBC is the most aggressive subtype and appears to be more common in premenopausal African and African-American women.[2,15,16] Histologically, such cancers are characterized by a high mitotic rate, pushing margin, solid architecture, lymphocytic infiltrates, increased nuclear grade, central fibrosis, and geographic necrosis.[10,14] Basal-like breast carcinomas are less likely to metastasize to the axillary lymph nodes and bone but have a propensity to involve the brain and lungs through the hematogenous spread.[14] In addition, several studies have documented immunohistochemistry as a surrogate to identify these subtypes of breast carcinomas.[14,16] This study is aimed at analyzing the clinicopathological features and IHC expression of basal-like phenotype in TNBC at Uyo, as well as stratifying of invasive breast carcinomas into main molecular subtypes and correlating the basal-like breast carcinoma subtype with the size of tumor, grade, and age of patient in the University of Uyo Teaching Hospital (UUTH), Uyo, Akwa Ibom State, Nigeria.
MATERIAL AND METHODS
Background of the study area
Akwa Ibom State is one of the states in the South-South geopolitical zone of Nigeria with an estimated population of 3,902,051 people and four major ethnic, groups namely, Ibibio, Annang, Oron, and Eket. The UUTH is the only tertiary health-care facility in the state and, therefore, is a major referral center for patients within Akwa Ibom State and, to some extent adjoining states such as Abia, Cross River, and Rivers States.
Study design
This study is a descriptive cross-sectional hospital-based study carried out with formalin-fixed paraffin-embedded tissue blocks from the Histopathology Department of UUTH, Uyo, Nigeria. These tissue blocks were taken to the National Hospital, Abuja, for IHC staining.
Study population and duration
All previously diagnosed and histologically confirmed cases of invasive breast carcinomas were received in the Department of Histopathology from July 01, 2016, to August 31, 2018.
Sample size determination
The sample size is calculated using Cochran’s formula for calculating the sample size for the prevalence studies.[17] n = Z2pq÷d2 where n = desired sample size, p = prevalence, q = 1-p, z = 95% confidence interval (1.96), and d = degree of accuracy desire (0.1%).
Using a prevalence of 25% (0.25) and substituting the above parameters in the formula, we have n = {1.962 × 0.25 (1–0.25)} ÷ 0.12 = 72.
Sampling method
The sampling method used in this study was purposive sampling.
Inclusion criteria
The cases included in this study were tissue blocks of histologically confirmed invasive breast carcinoma in the Histopathology Department of the UUTH between July 1, 2016, and August 31, 2018.
Exclusion criteria
Those excluded include all cases where tissue blocks could not be retrieved, all malignant cases with a history of neoadjuvant chemotherapy, radiotherapy, and other prior treatment for breast cancer, and all cases where the biopsy specimen was insufficient for IHC evaluation.
Materials
The patients’ histopathology request forms were retrieved to collect the biodata, tumor grade, and morphological diagnosis. All the formalin-fixed paraffin-embedded tissue blocks that fulfill the inclusion criteria were retrieved from the archives.
Methods
Formalin-fixed paraffin-embedded tissue blocks of invasive breast carcinomas diagnosed in the department within the study period were used for this study. Fresh sections from consecutive invasive breast carcinoma tissue blocks that meet the inclusion criteria were made, and immunostaining was done using monoclonal antibodies for ER, PR, HER-2, KI67, CK5/6, and epidermal growth factor receptor (EGFR) proteins. The immunohistochemistry protocol used was the Avidin biotin complex method. The antibody dilution factor used was 1:100 dilutions for all the antibody markers. The processed tissues were sectioned at two microns on the rotary microtome, and positive control slides were used within each batch of slides. Visual estimation for ER/PR immunohistochemically stained slides was done, and the percentage and intensity of tumor cells showing nuclear immunoreactivity was noted. Cases were considered positive for ER and PR by applying the standardized Allred scoring system, which is a semi-quantitative system that takes into consideration the proportion of positive cells (scored on a scale of 0–5) and staining intensity (scored on a scale of 0–3). The proportion and intensity of stained tumor nuclei were combined to produce total scores of 0 or 2 through 8. A score of 0–2 was taken as negative, while 3–8 was read as positive.[18] Membrane staining for HER-2 was scored using the College of American Pathologists (CAP) Guidelines as 0, 1+, 2+, and 3+ depending on the percentage of cells stained and the extent of membrane reactivity. Scores 0 or +1, 2+, and +3 were considered negative, equivocal, and positive, respectively.[18] The American Society of Clinical Oncology/CAP guidelines recommended fluorescence in situ hybridization (FISH) confirmation of all cases judged to be 2+ (equivocal) by immunohistochemistry. This was not done in this study for reasons of lack of the facility and resources in the country. For CK5/6, immunoreaction was positive when brown, homogenous cytoplasmic, or membranous staining of tumor cells was seen. Immunostaining in <10% of tumor cells was regarded as negative, while staining in >10% was considered positive. Membrane staining for EGFR was ascertained, and assessment on positivity and negativity was based on the percentage and extent of the membrane staining in the tumor cells.[14,19,20] Clear membrane staining was scored positive for EGFR. A semi-quantitative method was used to generate a score for each tumor tissue sample: The percentage of positive tumor cells in each slide (0– 100%) was multiplied by the dominant intensity pattern of staining (1 for negative, 2; weak; 3, moderate; and 4, intense). The overall scores ranged from 0 to 400 and were classed as negative, intermediate, and high if scored (0–200), (201–300), and (301–400), respectively.[20,21] Intermediate and high groups were considered positive. Only nuclear staining for Ki-67 was considered positive. The Ki-67 score was derived by ascertaining the percentage of positively stained cells among the total number of invasive cells in multiple fields. A cutoff of 14% was used to differentiate between Luminal A and Luminal B subtypes.[20,21]
Data collection and technique
The laboratory request forms, histopathology, and IHC results were reviewed to collect the age, menopausal status, morphological diagnosis, and grade of the carcinoma. IHC staining for ER, PR, HER-2, Ki67, CK5/6, and EGFR was done on the selected blocks as described above, and a combination of the different staining patterns used to classify the different molecular subtypes as follows: Luminal A (ER-positive and/or PR-positive, HER-2-negative, and Ki67 <14%); Luminal B (ER-positive and/or PR-positive, HER-2-negative, Ki67 >14% or ER-positive and/or PR-positive, and HER-2-positive); HER-2 enriched (ER-negative, PR-negative, HER-2-positive); basal-like (ER-negative, PR-negative, HER-2-negative, CK5/6-positive, and/or EGFR-positive); and normal-like/unclassifiable (ER-negative, PR-negative, HER-2-negative, CK5/6-negative, and/or EGFR-negative).
Data analysis
The data were collated using Microsoft Excel and analyzed using the Statistical Package for Social Sciences version 16 (SPSS 16). The results were presented as tables, graphs, and photomicrographs. Tests for association were done using Chi-square. The level of significance was set at P < 0.05.
Ethical consideration
Ethical approval to conduct the study was obtained from the Health Research and Ethics Committee of the UUTH (UUTH/AD/S/96/VOLXIV/540). The cost of the research was fully borne by the researchers.
RESULTS
Prevalence of breast carcinoma
A total of 443 breast tissue samples were received within the study period. Of this number, 227 (51.2%) were benign lesions, while 216 (48.8%) were carcinomas. This gave a frequency of 48.8%.
Demographic and histological characteristics
A total of 79 histologically confirmed invasive breast carcinomas were used for this study. The age range of the patients was 24–76 years, with a mean age of 43.96 ± 9 years. All the cases were from females. The premenopausal age group (24–44 years) accounted for 45.7% of cases. Majority of the invasive carcinomas were of no special type (72 cases, 91.1%), while the special subtypes accounted for 7 cases (8.9%). Most of the carcinomas were grade 2 (moderately differentiated), accounting for 58.2%. Grade 1 (well differentiated) and grade 3 (poorly differentiated) accounted for 11 (13.9%) and 22 (27.8%) of cases, respectively. The tumor size ranged from 1.5 to 10.0 cm with a mean age of 3.8 ± 1.9 cm [Table 1].
| Variables | Groups | Frequency | Percentage |
|---|---|---|---|
| Age group | 24–34 | 21 | 26.6 |
| 35–44 | 23 | 29.1 | |
| 45–54 | 23 | 29.1 | |
| 55–64 | 5 | 6.3 | |
| ≥65 | 7 | 8.9 | |
| Total | 79 | 100 | |
| Tumor size | 1.5–3.0 | 44 | 55.7 |
| 3.1–6.0 | 26 | 32.9 | |
| ≥6.1 | 9 | 11.4 | |
| Total | 79 | 100 | |
| Histologic types | Invasive carcinoma (NST) | 72 | 91.1 |
| Micropapillary | 2 | 2.5 | |
| Tubular | 2 | 2.5 | |
| Mucinous Ca | 3 | 3.8 | |
| Total | 79 | 100 | |
| Grades | Grade 1 | 11 | 13.9 |
| Grade 2 | 46 | 58.2 | |
| Grade 3 | 22 | 27.8 |
NST: No special type
IHC characteristics
Out of the 79 cases, ER and PR were positive in 42 (53.2%) and 26 (32.9%) of cases, respectively. HER-2 was positive in 10 (12.7%) of cases. Four (5.1%) were equivocal for HER-2 and, therefore, were excluded in subsequent phenotypic classification since they were not analyzed further with FISH. CK5/6 and EGFR were also positive in 13 (16.5%) and 9 (11.4%) of cases, respectively. Ki67 was high (>14%) in 21 (26.6%) of cases [Table 2, Figures 1-4].
| Markers | Negative (%) | Positive (%) | Equivocal (%) |
|---|---|---|---|
| ER | 37 (46.8) | 42 (53.2) | |
| PR | 53 (67.1) | 26 (32.9) | |
| HER-2 | 65 (82.3) | 10 (12.7) | 4 (5.1) |
| EGFR | 70 (88.6) | 9 (11.4) | |
| CK5/6 | 66 (83.5) | 13 (16.5) | |
| <14 | >14 | ||
| Ki67 | 58 (73.4) | 21 (26.6) |
PR: Progesterone receptor, ER: Oestrogen receptor, HER2: Human epidermal receptor, EGFR: Epidermal growth factor receptor
- Photomicrograph of invasive carcinoma no special type showing estrogen receptor nuclear-positive staining (Original magnification ×100).
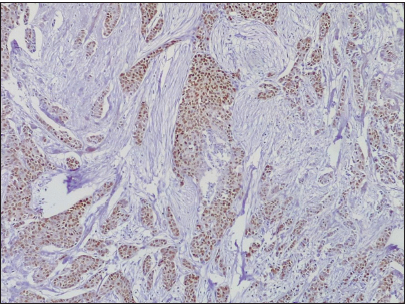
- Photomicrograph of invasive carcinoma no special type, showing progesterone receptor-positive reaction (Original magnification ×40).
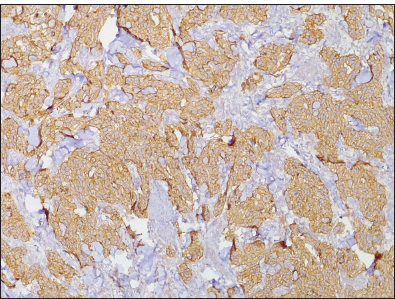
- Photomicrograph of invasive ductal carcinoma showing Her-2+++ (Original magnification ×40).
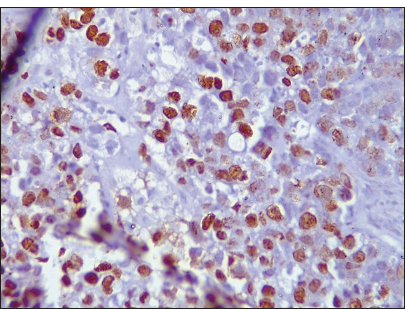
- Photomicrograph of invasive breast carcinoma no special type showing ki67 nuclear reactivity (Original magnification ×100).
Phenotypic classification based on immunohistochemistry
The majority of the breast carcinoma subtypes were Luminal A subtype, accounting for 34 (45.3%) cases; this is followed by the Luminal B subtype occurring in 10 (13.3%) cases [Table 3].
| TEST | No Tested | Positive (%) |
|---|---|---|
| LA | 75 | 34 (45.3) |
| LB | 75 | 10 (13.3) |
| HER-2EN | 75 | 4 (5.3) |
| Basal-Like | 75 | 13 (17.3) |
| Unclassified | 75 | 14 (18.7) |
LA: Luminal A, LB: Luminal B, HER2EN: Human epidermal receptor2 Enriched
TNBCs and basal-like subtype
Twenty-seven (36.0%) of the cases were TNBCs. The basal markers (CK5/6 and EGFR) were positive in 13 out of the 27 TNBCs (48.1%) representing predominant subtypes of TNBC and represented 17.3% of total number of non-equivocal immuno reportable breast carcinomas. This entailed 4 TN/EGFR-positive breast carcinoma cases (14.8%) and 8 TN/CK5/6-positive cases (29.6%). One (3.7%) of the TNBCs was positive for both CK5/6 and EGFR tumor markers [Table 4, Figures 5 and 6].
| HER2 | ER | PR | Total | |
|---|---|---|---|---|
| Negative (%) | Positive (%) | |||
| Negative | Negative | 27 (36.0) | 3 (4.0) | 30 (40.0) |
| Positive | 18 (24.0) | 17 (22.7) | 35 (46.6) | |
| Positive | Negative | 3 (4.0) | 2 (2.7) | 5 (6.7) |
| Positive | 2 (2.7) | 3 (4.0) | 5 (6.7) | |
| Total | 50 (66.7) | 25 (22.3) | 75 (100) | |
PR: Progesterone receptor, ER: Estrogen receptor, HER2: Human epidermal receptor
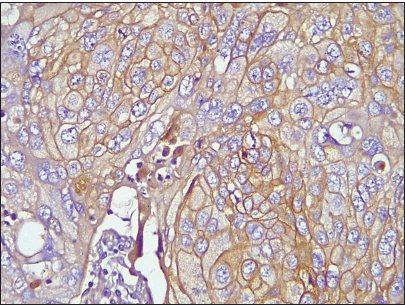
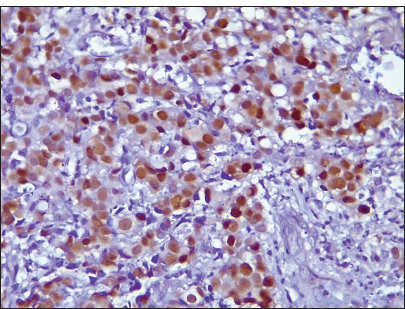
- Photomicrograph of invasive breast carcinoma no special type showing epidermal growth factor receptor membrane reactivity (Original magnification ×100).
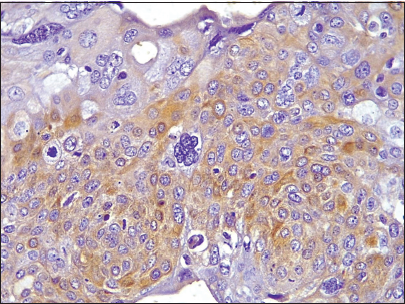
- Photomicrograph of invasive breast carcinoma no special type showing CK5/6 cytoplasmic reactivity (Original magnification ×100).
Correlation between basal-like phenotype and grade, tumor size, and age
The study showed that nine out of 13 basal-like breast carcinomas were grade 3 (poorly differentiated) it; observed that the tumor grade differs significantly in relation to the proportion of basal-like phenotype; those with poorly differentiated breast carcinoma having a higher proportion of basal-like breast carcinoma phenotype (P = 0.002 < 0.05). Although most of the basal-like breast carcinoma phenotypes were seen in the 35–44 years age group (31.8%), there was no statistical correlation between age and proportion of basal-like breast carcinoma phenotypes [Table 5].
| Variable | Group | NO. | Basallike breast carcinoma | P-value | OR (95%CI) |
|---|---|---|---|---|---|
| Grade | WD | 11 | 0 (0) | 0.002 | |
| MD | 43 | 4 (9.3) | 1 | ||
| PD | 21 | 9 (42.9) | 7.3 (1.91–28.0) | ||
| Tumor size (cm) | 1.5–3.0 | 42 | 7 (16.7) | 0.91 | |
| 3.1–6.0 | 24 | 4 (16.7) | |||
| ≥6.1 | 9 | 2 (22.2) | |||
| Age groups | 24–34 | 20 | 3 (15.0) | 0.12 | |
| 35–44 | 22 | 7 (31.8) | |||
| 45–54 | 21 | 1 (4.8) | |||
| 55–64 | 5 | 0 (0) | |||
| ≥65 | 7 | 2 (28.6) |
WD: Well differentiated, MD: Moderately differentiated, PD: Poorly differentiated, OR: Odds ratio, CI: Confidence interval
DISCUSSION
Breast carcinoma is the leading cancer in females with a highly heterogeneous behavior regarding its morphology, molecular genetics, biology, as well as clinical outcomes with or without treatment.[1-3] Hence, the classification of breast carcinomas histologically and immunohistochemically is imperative to prognosticating and predicting the response to therapy, though with limitations that might be overcome by the use of gene expression profiling.[4-16]
The index study showed that the frequency of breast cancers in Uyo, South-South Nigeria, was 48.8%; this compares relatively with a finding of 44.3% in a previous study in Uyo, as well as 45.8% in Makurdi, North Central Nigeria, 43.7% in Ebonyi, South East Nigeria and 47.9% in Sokoto, North West Nigeria, respectively.[22-25] Outside Nigeria, very low and high rates of 11.8% and 54.8% were reported in Pakistan and India, respectively.[26,27] From the foregoing, it is obvious that the prevalence of breast cancers differs with geographical location, probably due to the interplay of racial and cultural factors in the development of this disease.
On the basis of immunohistochemistry, this study showed that most of the breast carcinomas expressed ER positivity (53.2%) and PR positivity (32.9%). This is similar to the increased positivity expression of ER (65.1%), PR (54.7%) and ER (54.2%), PR (50.0%) reported by Adebamowo et al. and Nwafor and Keshinro in Ibadan and Lagos, respectively.[28,29] Concordant results of increased hormone receptor expression were also reported by Salhia et al. in Egypt and Carey et al. in North Carolina, USA, where positivity rates of ER (65.0%), PR (43.8%) and ER (60.0%), PR (50.0%) were reported, respectively;[15,30] although these values were higher than what was obtained in the index study. In contrast, studies conducted in Ghana and India showed low rates of ER+ tumors of 32.1% and 36.5%, respectively.[3,31] On the other hand, the index study showed that HER-2 was positive in 12.7% of cases; this compares with the results of 2.4%, 10.6%, 11.0%, and 25.5% reported by Rao et al. in India, Ukah et al. in Nnewi, South East Nigeria, Roy and Othieno. in Uganda, and Seshie et al. in Ghana, respectively.[31-34]
These variations in immunohistochemistry results from region to region could probably be explained by improper specimen collection and preservation, improper tissue processing, variable IHC techniques, interpretation, and scoring. Five molecular breast carcinoma subtypes were identified in this study. In decreasing order, the 75 breast carcinomas were distributed into Luminal A, Luminal B, basal-like, normal like/unclassified, and HER-2 enriched subtypes in 34 (45.3%), 10 (13.3%), 13 (17.3%), 14 (18.7%), and 4 (5.3%), respectively. This indicates that the Luminal A breast carcinoma phenotype accounted for the majority of the molecular phenotypes in this study. A concordant result of 43.9% was obtained by Ukah et al. in Nnewi, South East Nigeria.[34] Similar trends were also reported by Omoruyi et al. in Calabar, South South Nigeria, Bennis et al. in Morocco, Ihemelandu et al. in the USA, and Ozmen. In Turkey, where Luminal A constituted 52.4%, 53.6%, 55.4%, and 62.0% of breast carcinomas,[35-38] although the values are higher than the 45.3% in the index study. Preat et al., in their study on immigrant Arab women and European women, noted that the Luminal A subtype was more common in European women (46.0%).[39] In contrast, lower prevalence rates of 14.5% and 3.9%, 20.0%, and 25.6% for Luminal A subtype have been reported in Brazil, Saudi Arabia, Ilorin North Central Nigeria, and Ghana, respectively.[22,40-42] Luminal B breast carcinoma phenotype accounted for 13.3% of all cases in the index study. This differs from rates of 2.6%, 6.0%, 12.9%, 11.0%, 12.2%, and 11.8% recorded in Ibadan, Nnewi, Calabar, Ilorin, Ghana, and USA, respectively.[29,32,34,35,37,42] but contrasts with higher prevalence rates of 44.6%, and 56.0% reported in Brazil and Morocco, respectively.[39,40] The wide variations in the prevalence of the Luminal breast carcinoma phenotypes could be due to the use of different combinations of IHC markers in defining them. These include deployment of positivity or negativity of ER, PR, and HER-2 protein with or without positivity of Ki-67 ranging from 1% to 20%, as well as variable IHC pre-analytic and analytic study protocols influencing the immunohistochemistry result.[24,31] In addition, the biology of the tumors, as well as the genetic differences among different ethnic and racial, groups, has been shown to influence the expression pattern of tumors.[30,38] HER-2-enriched breast carcinoma phenotype accounted for 5.3% of the cases in the index study. This is, however less than the observed varying rates from 8.2% to 22.1% in some previous studies.[28,32] The difference in prevalence of HER-2-enriched breast carcinoma subtype could be adduced to the strong influence of pre-analytical variables such as cold ischemic time, period of fixation, and type of fixative as well as differences in the biology of tumors and criteria used in the IHC interpretation of the tumor markers. The basal-like breast carcinoma phenotype constituted 17.3% in this present study. This is lower than the rates of 21.2%, 26.5%, 33.1%, and 40.7% reported in the USA as well as in Calabar, Lagos, Ife and Nnewi, and Nigeria, respectively.[28,34,35,37] However, other studies have reported lower prevalence rates of 11.3%, 12.6%, and 10% in Egypt, Morocco, and Saudi Arabia, respectively.[30,36,41] Carey et al. reported that basal-like breast carcinoma phenotypes are seen more in premenopausal African-American (39.1%) when compared to postmenopausal African-American (14.1%).[15] The basal-like breast carcinoma phenotypes sometimes equate TNBCs, which were originally defined by gene expression profiling.[2,34,37,43]
Immunohistochemistry is used as a surrogate, employing different basal markers in addition to ER, PR, and HER-2. In this study, TNBC accounted for 36.0% of the cases. This is higher than 29.2%, 15.1%, 17.1%, and 15.0% reported in Lagos, Southwest Nigeria, Thailand, Brazil, and India, respectively.[28,31,38,40] In contrast, much higher rates were reported in Nnewi, Ghana, and India, where rates of 40.7%, 49.4%, and 50.0% were recorded, respectively.[31,32,34] The differences in the prevalence of TNBC could be due to the effect of pre-analytical variables as well as to the scoring system applied in interpreting the immunohistochemistry result. Furthermore, the ethnic, racial, and genetic differences in individuals and the biology of tumors can account for the differences in prevalence.
Although TNBC may be regarded as Basal-like subtype which could be distinguished by a wide variety of basal markers like CK5, CK5/6, EGFR, and CK14 [19,44,45], there is still no universally accepted immunohistochemical (IHC) definition of Basal-like breast carcinoma. A five marker model as proposed by Nielsen et al. (ER, PR, HER-2, CK5/6, and EGFR) is seen as the best combination of markers to further characterize this subtype where basal-like cancers are defined as those lacking both ER, PR, and HER-2 expression and expressing CK5/6 and/or EGFR.[19] This panel has a specificity of 100% and a sensitivity of 76% for the identification of basal-like tumors.[19] The index study showed 13 out of the 27 TNBC to be either EGFR+ or CK5/6 or both, giving a basal-like breast carcinoma frequency of 48.1%. Gazinska et al. reported a frequency of 47.9%, which is similar to this index study.[14] However, Rakha et al in UK and Reang et al. in India reported a higher frequency of 72.4% and 71%, respectively.[46,47] In contrast to the above, Cheang et al. recorded a low prevalence of 9.0%.[48] This wide variation may be due to the nonstandardization of the various combinations of tumor markers that are used in identifying the basal-like subtype as well as the difference in the biology of tumors.
This study showed that nine out of 13 basal-like breast carcinomas were grade 3, with a positive association between this tumor phenotype and high tumor grade. This is comparable to similar studies by Zakaria et al., Gazinska et al., and Cheang et al., which showed that basal-like breast cancers are associated with high-grade carcinomas.[14,20,21] In contrast, a study conducted in Egypt did not find any association between the tumor grade and breast cancer molecular subtype.[30]
Limitations of the study
The absence of facilities for FISH did not permit proper classification of equivocal cases of HER-2.
CONCLUSION
Our study confirmed that the Luminal A subtype of breast carcinomas was the most commonest, and there was an appreciable number of the basal-like breast carcinoma subtype in Uyo with a frequency of 48.1% among the TNBCs.
Acknowledgments
We are eternally grateful to the Almighty God, Management, and staff of the University Teaching Hospital for the success of this study. We also appreciate the effort of Mr. Madukwe Jonathan from National Hospital Abuja for providing the technical inputs and staining of the immuno slides.
Ethical approval
The research/study is approved by the Health Research and Ethics Committee of the UUTH, number (UUTH/AD/S/96/VOLXIV/540), dated 22nd June 2016.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Molecular and cellular heterogeneity in breast cancer. Am J Pathol. 2013;183:1113-24.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular portraits of human breast tumours. Nature. 2000;406:747-52.
- [CrossRef] [PubMed] [Google Scholar]
- GLOBOCAN 2012 cancer incidence and mortality worldwide: IARC cancer base France: IARC; 2013.
- [Google Scholar]
- The current state of breast cancer classification. Ann Oncol. 2012;23:207-10.
- [CrossRef] [PubMed] [Google Scholar]
- Classification and prognosis of invasive breast cancer; from morphology to molecular taxonomy. Mod Pathol. 2010;23:60-4.
- [CrossRef] [PubMed] [Google Scholar]
- Issues and updates: Evaluating oestrogen receptor-? progesterone receptor, and HER2 in breast cancer. Mod Pathol. 2010;23:S52-9.
- [CrossRef] [PubMed] [Google Scholar]
- Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J Clin Oncol. 2014;5:412-24.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of immunohistochemistry in breast cancer. World J Clin Oncol. 2014;5:382-92.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular stratification of triple-negative breast cancer. Oncologist. 2010;15:39-48.
- [CrossRef] [PubMed] [Google Scholar]
- Triple-negative breast cancer: Clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010;23:123-33.
- [CrossRef] [PubMed] [Google Scholar]
- Gene expression patterns of breast carcinomas distinguish tumour subclasses with clinical implications. Proc Natl Acad Sci. 2001;98:10869-74.
- [CrossRef] [PubMed] [Google Scholar]
- Subtyping of triple-negative breast cancer: Implications for therapy. Cancer. 2015;121:8-16.
- [CrossRef] [PubMed] [Google Scholar]
- Triple-negative breast cancer. Asian Pac J Cancer Prev. 2014;15:2427-31.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of basal-like triple-negative breast cancer defined by morphology, immunohistochemistry and transcriptional profiles. Mod Pathol. 2013;26:955-66.
- [CrossRef] [PubMed] [Google Scholar]
- Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492-502.
- [CrossRef] [PubMed] [Google Scholar]
- Population differences in breast cancer: Survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27:4515-21.
- [CrossRef] [PubMed] [Google Scholar]
- Research methodology with statistics for health and social sciences Ilorin: Nathadex Publishers; 2004.
- [Google Scholar]
- Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch Pathol Lab Med. 2014;138:595-601.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367-74.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical characterization and subclassification of triple-negative breast cancer of Egyptian female patients. Med J Cairo Univ. 2018;86:2533-41.
- [CrossRef] [Google Scholar]
- Ki67 index, HER-2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736-50.
- [CrossRef] [PubMed] [Google Scholar]
- Histological characteristic of breast lesions in Uyo. Niger J Surg. 2018;24:76-81.
- [CrossRef] [PubMed] [Google Scholar]
- The spectrum of breast diseases in Nigeria North Central: A histpathologic survey. J Adv Med Pharm Sci. 2017;13:1-6.
- [CrossRef] [Google Scholar]
- Clinocopathologic study of breast lump in Abakaliki, South Eastern Nigeria. Asian J Med Sci. 2016;7:58-63.
- [Google Scholar]
- Clinco-pathological profile of patients with breast disease. Diagn Pathol. 2013;8:77.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern of hormone receptors and human epidermal growth factor receptor 2 status in subSaharan breast cancer cases: Private practice experience. Niger J Clin Pract. 2015;18:553-8.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistcochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat. 2008;110:183-8.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular subtype analysis determines the association of advanced breast cancer in Egypt with favorable biology. BMC Womens Health. 2011;11:44.
- [CrossRef] [PubMed] [Google Scholar]
- Morphological profile and receptor status in breast carcinoma: An institutional study. J Cancer Res Ther. 2013;9:44-9.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective analysis of breast cancer subtype based on ER/PR and HER2 status in Ghanaian patients at the Korle Bu Teaching Hospital, Ghana. BMC Clin Pathol. 2015;15:14.
- [CrossRef] [PubMed] [Google Scholar]
- Breast carcinoma in Uganda: Microscopic study and receptor profile of 45 cases. Arch Pathol Lab Med. 2011;135:194-9.
- [CrossRef] [PubMed] [Google Scholar]
- The immunohistochemical profile of breast cancer in Indigenous women of South East Nigeria. Ann Med Health Sci Res. 2017;7:83-7.
- [Google Scholar]
- Prevalence of molecular subtypes of breast carcinoma in University of Calabar Teaching Hospital using Immunohistochemistry as surrogates for Intrinsic DNA gene characteristics. J Dent Med Sci. 2018;17:64-73.
- [Google Scholar]
- Prevalence of molecular subtypes and prognosis of invasive breast cancer in north-east of Morocco : retrospective study. BMC Res Notes. 2012;5:436.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: Age specific prevalence and survival. J Surg Res. 2007;14:109-18.
- [CrossRef] [PubMed] [Google Scholar]
- Breast cancer in Turkey: Clinical and histopathological characteristics (analysis of 13.240 patients) J Breast Health. 2014;10:98-105.
- [CrossRef] [PubMed] [Google Scholar]
- Differences in breast carcinoma immunohistochemical subtypes between immigrant Arab and European women. Diagn Pathol. 2014;9:26.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular breast cancer subtypes and therapies in a public hospital of Northeastern Brazil. BMC Womens Health. 2014;14:110.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular classification of breast cancer: An overview with emphasis on ethnic variations and future perspective. Saudi J Med Med Sci. 2013;1:14-9.
- [CrossRef] [Google Scholar]
- Molecuar profiles of breast cancer in Ilorin, Nigeria. J Clin Oncol. 2010;28:1602.
- [CrossRef] [Google Scholar]
- Breast cancer classification according to immunohistochemical markers : Clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol. 2009;20:628-35.
- [CrossRef] [PubMed] [Google Scholar]
- Basal-like breast cancer: A critical review. J Clin Oncol. 2008;26:2568-81.
- [CrossRef] [PubMed] [Google Scholar]
- Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5-23.
- [CrossRef] [PubMed] [Google Scholar]
- Triple-negative breast cancer: Distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009;15:2302-10.
- [CrossRef] [PubMed] [Google Scholar]
- Immunophenotypic and morphological profile of basal-like and non-basal-like invasive breast carcinoma. Natl J Lab Med. 2007;6:10-5.
- [Google Scholar]
- Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368-76.
- [CrossRef] [PubMed] [Google Scholar]







