Translate this page into:
Pathobiological bases of asthma-COVID-19 interaction: A theoretical viewpoint

*Corresponding authors: Hayatu Saidu, Department of Medical Laboratory Science, Bayero University Kano, Kano State, Nigeria. hayatuddeensaeed@gmail.com/hsaidu.mls@buk.edu.ng to hayatu saidu
Jamilu Abubakar Bala, Depatment of Medical Laboratory Science, Bayero University, Kano, Kano State, Nigeria. jamiluwudil@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Saidu H, Aliyu IA, Gwarzo MY, Musa BP, Bala JA, Adeiza MA, et al. Pathobiological bases of asthma-COVID-19 interaction: A theoretical viewpoint. Adesh Univ J Med Sci Res 2022;4:56-66.
Abstract
Patients with asthma are susceptible to viral respiratory infections, due to weakened cellular immunity, chronic airway inflammation, and some other reasons. In fact, asthma was found to be a comorbidity to the Middle East respiratory syndrome coronavirus infection. Moreover, some asthma therapies like systemic corticosteroids used to manage severe asthma were found to be associated with severe acute respiratory syndrome coronavirus (SARS-CoV) viremia in the previous pandemic. However, from the epidemiological studies conducted so far across the globe, asthma patients are not exceptionally susceptible to COVID-19 compared to the general public; as opposed to the association seen with chronic obstructive pulmonary disease, diabetes, hypertension, obesity, and other known comorbidities of COVID-19. The bases for this interaction are not adequately understood. However, the heterogeneity of asthma disease as it relates to its various endotypes, altered angiotensin-converting enzyme (ACE2) expression in the airways, effect of rhinoviral infections, and effect of inhaled corticosteroids and biological response modifiers (BRMs), are the proposed mechanisms behind this interaction. Increased activity of ADAM 17 as induced by interleukin-13 at apical portion of pneumocytes may be responsible for the shedding of ACE2 on asthmatic airways. Furthermore, inhaled corticosteroids may prevent the occurrence of acute lung injury and, hence, acute respiratory distress by transrepression of pro-inflammatory pathways and transactivation of anti-inflammatory pathways. The antiviral effects of some inhaled corticosteroids whose molecular targets are not known may involve downregulation and competitive binding to the chaperone proteins heat shock proteins 90 and 70 between glucocorticoid receptor and nucleoprotein of SARS-CoV-2. MEDLINE was searched for terms such as asthma and COVID-19, antiviral effects of inhaled corticosteroids, BRM, and mechanisms of asthma-COVID-19 interaction. The reference lists of the obtained articles were also searched for additional literature.
Keywords
Asthma
COVID-19
Proposed pathobiologic mechanisms
INTRODUCTION
The 2022 update of the Global Strategy for Asthma Management and Prevention (GINA) has defined asthma as “a heterogeneous disease characterized by airway inflammation typified by clinical manifestations such as cough, wheezing, chest tightness, and shortness of breath that vary over time and in intensity, together with variable expiratory airflow limitations.”[1] Around 300 million individuals are currently affected, this may increase to 400 million by 2025.[2] COVID-19 is an infectious disease caused by the severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2), the disease is characterized by myriad of respiratory symptoms and multiorgan dysfunction.[3,4] The disease started in 2019 and reached pandemic level the same year, infecting millions of people and claiming millions of lives.[4] Both asthma and COVID-19 are diseases of the lower respiratory tract. Comorbidities have been observed to potentiate the pathogenesis of COVID-19, giving rise to a more severe disease.[5] The relationship between viral infections and asthma is well-established, with more than 80% of acute asthma exacerbations triggered by viral infections of the respiratory tract.[6] Furthermore, asthma is regarded as a risk factor for the development of viral lower respiratory tract infections.[7] Of the respiratory comorbidities associated with COVID-19, it is observed that patients with chronic obstructive pulmonary disease are at a higher risk of having severe COVID-19.[8] As such, in the early phase of the pandemic, a similar relationship was predicted between asthma and COVID-19.[9] However, epidemiological data are not in keeping with this postulate, as no high burden of severe COVID-19 is observed for now among patients having asthma as comorbidity.[10] This observation has been surprising to allergists, immunologists, and pulmonologists. A reduced expression of the receptor for SARS-CoV-2 on the respiratory epithelium of patients with asthma, antiviral effects of corticosteroids used in asthma management, and other biological response modifiers (BRMs) among others are proposed to be mechanisms behind the normal burden of COVID-19 among patients with asthma. This review endeavors to give a concise account of the proposed mechanisms behind the reduced incidence of severe COVID-19 among patients with asthma as comorbidity.
VIRAL INFECTIONS AND PATHOGENESIS OF ASTHMA
Acute attacks of asthma are triggered by a myriad of factors; the relevance of the triggers varies with the biological endotype of asthma. Of note, among such factors are airway infections due to rhinoviruses, metapneumoviruses, parainfluenza viruses, respiratory syncytial viruses, and coronaviruses.[11] Clinical infection in early childhood with these viruses is associated with wheezing episodes and a proportion of these children develop asthma later in life.[12,13] Human rhinoviruses (HRVs) are the most important viral triggers of asthma compared to other viruses.[11] In patients with established asthma, viral respiratory tract infections are responsible for the vast majority of exacerbation cases.[11,14]
It is believed that viral infections tend to alter the hosts immune response; however, this alteration usually occurs in the settings of genetic susceptibility as made evident by the ubiquitous nature of rhinoviral infections in the general population.[15] Several studies have demonstrated reduced type one interferon production in patients with asthma compared to normal healthy controls [Table 1], and this is found to correlate well with increased viral replication and slow clearance of infection particularly due to HRV.[16,17] Altered T-cell responses characterized by suppression of adaptive cellular immunity to viruses and enhanced IgE antibody production can be seen.[16,18] Further investigations have demonstrated associations between genetic polymorphisms in the innate and adaptive immune pathways important in the fight against viral infections and the development and exacerbations of asthma. These polymorphisms have been replicated by several independent studies [Table 1].
| Author (s) | Research questions | Result | Role in the immune response | Effects in pathogenesis of asthma |
|---|---|---|---|---|
| Loisel et al., 2016[82] Li et al., 2007[83] |
STAT4 gene polymorphisms and asthma in humans | STAT4 rs4853546 SNP is associated with virus-induced asthma exacerbations STAT-4 T90089C is associated with increased susceptibility to asthma |
A signaling molecule important in interleukin-12 production. Important cytokine in Th1 polarization | Poor CD8+T-cell response to respiratory viruses |
| Loisel et al., 2016[82] Tabèze et al., 2019[84] |
JAK2 gene polymorphisms and asthma in humans | JAK2rs3780375 HRV-associated wheezing and asthma. JAK2 V617F associated with severe asthma | Signaling molecule important in GM-CSF production; a cytokine important in myelopoiesis[85] | Poor production of macrophages which play a role in antigen presentation and interferons production |
| Loisel et al., 2016[82] Ching et al., 2010[86] Hamano et al., 2005[87] |
M×1 gene polymorphisms and asthma in humans | Rs469390 HRV-associated wheezing and asthma. GG genotype is more frequent in hypoxemic patients with SARS-CoV infection |
M×1 is an interferon-inducible gene that interferes with viral nucleoprotein complex formation[88] | Impaired type one interferon-mediated antiviral state in host’s cells |
| Loisel et al., 2016[82] Einisman et al., 2015[89] Iordanidou et al., 2014[90] |
VDR gene polymorphisms in humans | VDR rs4328262 SNP is associated with high asthma burden and viral loss of control Apal variant is associated with less severe disease compared to Bsml and Fokl |
Plays role in T-cell development, differentiation, and function.[91] | Poor adaptive cellular immune response to viruses |
| Loisel et al., 2016[82] | DDX58 gene polymorphisms and asthma in humans | DDX58rs10813831SNP is associated with viral loss of asthma control and childhood asthma. | A RIG1 pattern recognition receptor that senses cytoplasmic viral RNA and induces type one interferon production[92] | Impaired antiviral interferons production |
| Loisel et al., 2016[82] | EIF2AK2 gene polymorphisms and asthma in humans | EIF2A rs4293920 is associated with prolonged HRV infection | It inhibits ternary tRNAMet-GTP-eIF2 complex formation there by stalling viral and hosts protein synthesis[93] | Enhanced replication of viruses in the airways |
HRV: Human rhinoviruses, GM-CSF: Granulocyte monocyte colony-stimulating factor
EPIDEMIOLOGY OF COVID-19 INFECTION AMONG PATIENTS WITH ASTHMA
The linkage between COVID-19 and asthma is still a subject of argument among experts. Across the globe, especially Europe, North America, and Asia, researches investigating asthma as a comorbidity in COVID-19 are still emerging. In most of these studies, the study designs were observational including prospective case series, cross-sectional design, case–control, and cohort design. Some of the studies reviewed investigated the overall burden of COVID-19 among patients having asthma, incidence of COVID-19 disease severity, effects of corticosteroids, and biologic response modifiers therapy on severity of COVID-19 in patients having asthma and mortality due to COVID-19 [Table 2]. Most of the studies reported minimal or no association between asthma and severe COVID-19 disease. [Table 2] summarizes the findings from some salient epidemiological studies on the association between COVID-19 and asthma.
| Authors | Study design | Relevant aims | Sample size | Region of the world | Outcome |
|---|---|---|---|---|---|
| Song et al., 2020[8] | Retrospective analytical study on two groups of COVID-19 patients, one with asthma as comorbidity and the other with COPD | Assess the role of asthma in development of severe COVID-19 | Twenty-one COPD and 22 asthma with a diagnosis of COVID-19 | Wuhan, China | Asthma patients have reduced risk of developing severe COVID-19 than patients with COPD (OR: 23.433; 95% CI 1.525–360.135;P<0.01)1 |
| Richardson et al., 2020[94] |
Prospective case series of patients with COVID-19 admitted to 12 hospitals | Identification of comorbidities to COVID-19 | 5700 patients with a confirmed diagnosis of COVID-19 | New York, USA | Only 479 (9%) patients have asthma, compared to hypertension (3026), obesity (1737), and diabetes (1808) |
| Li et al., 2020[95] | Ambispective cohort study of severe versus non-severe COVID-19 | Identification of comorbidities to COVID-19 | 548 patients with a confirmed diagnosis of COVID-19 of which 269 have severe disease | Wuhan, China | The prevalence of asthma, hypertension, diabetes, and coronary heart disease in the total (548) population is 0.9%, 30.3%, 15.1%, and 10%, respectively. It is 1.5%, 38.7%, 19.3%, and 10.4% among those with severe COVID-19 |
| Lee et al., 2020[96] | Retrospective cohort study | Association between asthma and respiratory failure and death due to COVID-19 | 686 asthma patients with COVID-19 compared with 6586 COVID-19 patients without asthma | South Korea | Asthma is not a significant risk factor for respiratory failure or mortality due to COVID-19; OR: 0.99, P: 0.997 and OR: 1.06, P: 0.759 |
| Wang et al., 2020[9] | Meta-analysis of original data on severe COVID-19 and asthma | Association of asthma and mortality due to COVID-19 | 744 asthmatic patients and 8151 non-asthmatic patients were compared2 | Asthma had no significant effect on mortality (OR=0.96; 95% CI 0.70–1.30; I2=0%;P=0.79)2 | |
| Bloom et al., 2021[44] |
Multicenter prospective cohort study comparing four cohorts of COVID-19 patients with the following comorbidities: Asthma, COPD, asthma+COPD, and COVID-19 patients without asthma or COPD3 | Characterization of respiratory comorbidities among patients with COVID-19, risk of mortality from COVID-19 due to comorbidity, and the effect of ICS on COVID-19 severity | 75,463 patients with COVID-19 | UK (Scotland and England) | The prevalence of asthma, COPD, and asthma+COPD is 10.41%, 13.6%, and 2.75%, respectively. Patients with asthma in the age range of 16–39 years (1867 with asthma vs. 7083 without asthma) and≥50 years (5918 with asthma vs. 59,735 without) have higher odds for critical care need OR: 1.20, 95% CI (1.05–1.37) and 1.17, 95% CI (1.08–1.27) |
| Schultze et al., 2020[53] |
Prospective cohort study | Occurrence of severe COVID-19 and mortality among asthma patients managed with Inhaled corticosteroids versus beta agonists | Two cohorts of 148,557 with COPD and 818,490 with asthma | UK | Asthma patients prescribed high-dose ICS were at increased risk of COVID-19-related death compared to those on beta-agonists, hazard ratio and 95% CI: (1•55 [1•10–2•18]) |
| Izquierdo et al., 2021[46] | Multicenter retrospective cross-sectional study | Assess the impact of asthma on COVID-19 | 71,182 participants with asthma | Spain | Only 1006 asthma patients (1.4%) have COVID-19, 26.1% required hospitalization, asthma patients with COVID-19 are older 55±20 (P=0.001), have more comorbidities burden than in those without COVID-19. OR (95% CI) 2.02 (1.78–2.30, 1.72 (1.49–1.98 and 1.72 (1.45–2.03) for HTN, dyslipidemia, DM, and obesity, respectively |
| Adir et al., 2021[47] | Case–control and cohort design | Assess association between corticosteroid and biologics use in asthma COVID-19 | All participants (80,602) were patients with asthma. 8242 with COVID-19 and 72,360 without COVID-19 | Israel | 10.2% have COVID-19. Biologics and corticosteroids are not associated with overall occurrence of COVID-19 (adjusted OR, 0.99; 95% CI, 0.73–1.33; for SCS use: adjusted odds ratio, 0.96; 95% CI, 0.90–1.03). However , chronic systemic corticosteroid use (≥6 prescriptions in the previous year) is an independent risk factor for severe COVID-19 (adjusted HR and 95% CI: 2.19 and 1.63–2.94) |
PROPOSED PATHOBIOLOGICAL MECHANISMS BEHIND LOW INCIDENCE OF COVID-19 AMONG PATIENTS WITH ASTHMA
Reduced angiotensin-converting enzyme 2 (ACE) expression in asthmatic airways
ACE2, a close homologue of ACE, is a transmembrane protein found on the surface of lung epithelial cells. It is also expressed on the cell surface by the kidney, heart, brain, and the intestines, respectively.[19,20] The predominant expression of the enzyme in the airways is on the sinonasal epithelium and the type 2 pneumocyte of the lungs[21,22] with the enzyme being much concentrated on the apical aspect of the type 2 pneumocyte.[22] Primarily, the enzyme functions to generate the angiotensin 1–7 from angiotensin II.[23] Angiotensin II is obtained from angiotensin I by the activity of ACE2.[23] Angiotensin 1–7 lowers blood pressure and prevents the profibrotic and hypertrophic effects of angiotensin II.[24]
Binding of the SARS-CoV-2 through the spike protein to the extra membranous domain of the ACE2 leads to the internalization of the virus particle through endocytosis.[25,26] The enzyme serves as receptor to both SARS-CoV-1 and SARS-CoV-2 and is indispensable for infection of pneumocytes.[4] Variability in the expression of the ACE2 receptor on respiratory epithelium across individuals and disease states has been reported.[5,20] Asthma modifies the expression of the ACE2 receptor on the respiratory epithelium.[14]
ACE2 is regulated in two ways in asthma, and the direction of the regulation depends largely on the asthma endotype. The endotypes include T helper cell type 2 (Th2)-mediated atopic eosinophilic asthma, T helper cell type 17 (Th17)-mediated neutrophilic asthma, paucigranulocytic asthma, and idiopathic eosinophilic asthma [27] [Figure 1]. The endotypes represent the pathobiological mechanisms of asthma disease and account for its heterogeneity.[2] In Th2 high/eosinophilic asthma, the cytokines interleukin (IL)-4 and IL-13 secreted by the innate lymphoid cell type 2 and the Th2 cell downregulate ACE2 expression. Thus, the reduced expression may deprive SARS-CoV-2 of its needed receptor for infectivity.[28,29] We suggest that IL-13 alters ACE2 expression by increasing the activity of ADAM-17 at the apical portion of the airway epithelial cells where the ACE2 is maximally expressed [Figure 2].[30,31] ADAM-17 enhances the shedding of ACE2 from the surface of pneumocytes.[32] IL-13 may also act by activating protein kinase C or extracellular signal regulated kinase; these two enzymes are known to activate ADAM-17 which cleaves ACE2 from cell surface.[29] In contrast, in Th17 high/neutrophilic asthma, the cytokine IL-17 can upregulate the expression of ACE2.[8] Coincidentally, the predominant asthma endotype is the Th2 atopic eosinophilic asthma[28] and this may explain why the reduced incidence of COVID-19 among patients with asthma. Furthermore, the Th2 high/eosinophilic asthma endotype is associated with eosinophilia; and elevated peripheral blood eosinophil count is regarded as good prognostic marker of COVID-19.[33,34] Furthermore, the expression of reduced affinity ACE2 has been reported to occur due to rhinoviruses infection.[35] As highlighted previously, rhinoviruses are linked to asthma exacerbations. Rhinoviruses induce the expression of a short isoform of ACE2 which has reduced affinity to SARS-CoV-2 spike protein thus inhibiting the entry of SARS-CoV-2 into pneumocyte.[35]

- Proposed pathobiologic mechanisms of interaction between asthma and COVID-19.
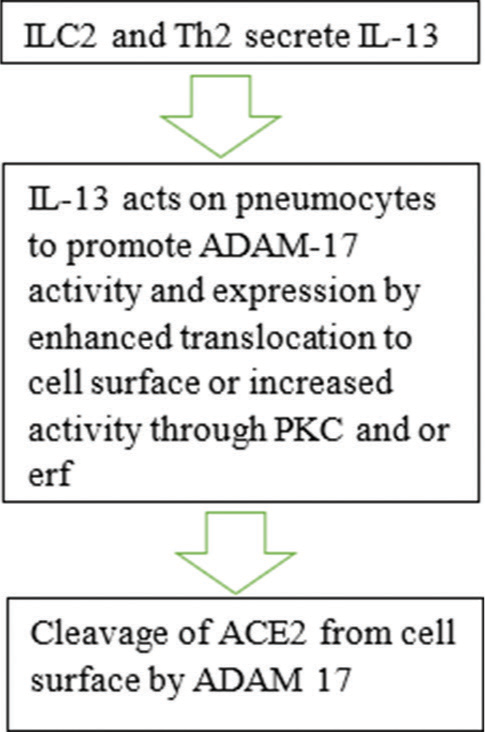
- Proposed effects of interleukin-13 (IL-13) on angiotensin-converting enzyme 2 (ACE-2) expression on asthmatic airways. PKC: Protein kinase C, Erf: Extracellular signal regulated kinase, ADAM-17: A disintegrin metalloprotease member 17.
Anti-inflammatory and antiviral effects of corticosteroids
Corticosteroids are used in the management of bronchial asthma.[1] The drugs counteract the allergic inflammation seen in asthma by reducing vascular permeability and hence laryngeal edema[36] minimizing the selective trafficking of leukocytes from the peripheral blood to the lungs by downregulating the expression of the cellular adhesion molecules selectin and integrins and interfering with the maturation and survival of polymorphonuclear leukocytes.[37-39] The relevance of corticosteroids in viral infections is incompletely understood and the available information has been contradictory. Early intravenous corticosteroids use was reported to have association with higher viral load and slower clearance of SARS-CoV infection among humans.[40-42] Inhaled corticosteroids use in asthma is associated with higher incidence of respiratory tract infections.[14]
The use of corticosteroids in the management of COVID-19 has had mixed reactions [Table 2].[43-48] In addition to the pharmacology of the various corticosteroids, differences in study design, variability in disease severity, choice, and interpretation of statistical methods among others could have contributed to the variations [Table 2]. Systemic corticosteroids are found to be effective in reversing hyperinflammatory acute respiratory syndrome seen in severe COVID-19.[49] In the UK RECOVERY trial, it was found that systemic corticosteroids (6 mg dexamethasone for 10 days) in COVID-19 patients hospitalized with severe disease and needing oxygen or respiratory/ventilatory support had reduced mortality compared to those that did not get dexamethasone.[50] Moreover, in vitro experiments have demonstrated that corticosteroids such as ciclesonide, algestone acetophenide, budesonide, and mometasone can inhibit the replication of the Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2, respectively.[51,52] Ciclesonide was found to act by inhibiting the non-structural protein 15 of SARS-CoV-2.[51] Conversely, however, systemic corticosteroids such as prednisone and prednisolone, used in the management of acute exacerbations of asthma, were found to have no effect on SARS-CoV-2 replication.[51] Many studies have identified varying effects on the use of corticosteroids in the management of asthma patients with COVID-19.[9,44,47,48,53] It is hypothesized that the anti-inflammatory and antiviral effects of inhaled corticosteroids are behind the reduced severity of COVID-19 among patients with asthma.[10] It was, however, observed that the worsening of COVID-19 following corticosteroids administration usually occurs among patients with severe asthma.[53]
The most notable manifestation of COVID-19 is acute respiratory distress syndrome (ARDS). ARDS occurs as an indicator of severe acute lung injury (ALI). ALI is mediated by local innate inflammatory mechanisms in the airways. Activation of resident pulmonary macrophages following pneumocyte injury by SARS-CoV-2 infection leads to release of innate inflammatory cytokines such as tumor necrosis factor-alpha (TNFα), interleukin 1, 6, and macrophage inflammatory protein-α.[54] TNFα induces microvascular endothelial activation characterized by increased expression of chemotactic molecules and procoagulant proteins. This leads to exodus of leukocytes into the alveolar spaces and thrombosis.[4] Interstitial mononuclear cell infiltration is also seen that the monomorphs may associate in consonance with polymorphs to orchestrate an immune complex mediated injury to the lung tissue characterized by complement system activation and hemostatic aberrations.[4,55] The polymorphs degranulate to release proteolytic enzymes, reactive oxygen and nitrogen species, and cytokines. The activity of polymorphs causes further damage to the pneumocytes and the endothelium. The microvasculature becomes leaky, leading to intra-alveolar fluid accumulation.[56] Damage to type 2 pneumocytes impedes surfactants production thus further compromising gaseous exchange and hence the fall in the percentage saturation of oxygen and the hypoxemia.[56] It is assumed that inhaled corticosteroids significantly counteract this vicious cycle in asthmatics by transrepression of inflammatory genes and transactivation of anti-inflammatory genes in the airways of asthmatics. As stated above, corticosteroids downregulate the migration of inflammatory cells and the release of inflammatory cytokines. Corticosteroids are lipophilic as such; they are quickly transported across the lipid bilayer of biological membranes.[57] On entry into the cell, corticosteroids (glucocorticoids [GCs]) are quickly bound to by a glucocorticoid receptor (GR) which is chaperoned to heat shock protein 90, 70, and other proteins. The formed complex dissociates from the chaperone proteins and is translocated into the nucleus assisted by import proteins α and 13.[58] The complex as a homodimer binds to promoter sequences (glucocorticoid response elements [GREs]) of responsive genes through the DNA-binding domain of the GR. Subsequently, recruitment of additional transcriptional cofactors leads to chromatin remodeling, RNA polymerase II binding, and gene expression.[59] In other situations, the GRE may induce repression of the glucocorticoid responsive genes. This is usually done by monomeric GC/GR, and it happens when the activity of other transcription factors such as nuclear factor-kappa beta and activation protein one is impeded by the GC/GR transcription factor through competition for binding space in the form of composite binding or tethering.[60,61] Through the mechanisms mentioned above, corticosteroids downregulate the expression of the following mediators: Interleukin 1β, 6, 8, 11, TNFα, granulocyte monocyte colony-stimulating factor, monocyte chemotactic protein-1, eotaxins, migration inhibitory protein-1, and regulated on activation expressed and secreted.[58] In these ways, inhaled corticosteroids counteract neutrophil and monocyte recruitment into airways, stabilize mast cells, and prevent the secretion of histamine and late phase lipid mediators by inhibiting phospholipase A2 through lipocortin induction.[62] It also inhibits fibroblast proliferation and collagen deposition.[62] Most of these processes and mediators are central to the pathogenesis of COVID-19 as stated above.[54]
As previously mentioned, the inhaled corticosteroids ciclesonide inhibits SARS-CoV-2 replication by blocking non-structural protein 15. The non-structural protein 15 is one of the 16 proteins which form the replicase machinery of SARS-CoV-2. These proteins are part of a larger protein, polyprotein 1ab which is encoded by ORF1ab of SARS-CoV-2. Non-structural protein 15 is a viral RNA modification enzyme with uridylate-specific endoribonuclease activity.[63] However, the antiviral target of mometasone is yet to be identified.[51] Mometasone was found to inhibit the replication of coronaviruses that have developed resistant mutation to ciclesonide; thus suggesting that mometasone has a different target from ciclesonide.[51] Furthermore, the targets of the other inhaled corticosteroids with antiviral activity are also not known.[51] Mometasone and possibly other inhaled corticosteroids with antiviral activity may work by targeting cellular heat shock proteins 70 and 90. Heat shock proteins are indispensable in maintenance of protein stability and cellular homeostasis. They are extensively used as chaperones to both host cell proteins and viral proteins. They protect the proteins against unfavorable conditions caused by stressors and are found in all cellular compartments, including the endoplasmic reticulum which serves as niche for SARS-CoV-2 replication.[63,64] Coronaviruses are found to utilize heat shock protein 90 for stabilization of nucleoprotein and protection against cellular proteasomes of the host.[65] Inhibition of HSP 90 drastically reduces MERS-CoV and SARS-CoV-2 in cell cultures.[65] Similarly, GC receptor utilizes heat shock proteins 70 and 90 as stabilizers in the cytosol. Thus, competition for the available heat shock proteins between viral and normal proteins including the GR is inevitable. It is found that cortisol induces the downregulation of heat shock protein 70 in fish.[66] Whether corticosteroids can alter the expression of heat shock proteins in humans is however not adequately studied. It was observed that stable binding of HSP 90 to geldanamycin disrupts glucocorticoid receptor’s stability and hence its ability to bind GC,[67] thus suggesting that a better competitor can make HSP 90 unavailable for even the physiological routines of the cell. Therefore, through such competition, GR may inhibit SARS-CoV-2 replication. The observation that ICS do not induce HSP 90 expression in the respiratory epithelium[68] and that serum level of HSP 90 is not altered by prednisolone administration[69] may further support this view. However, there are contrary observations on the effects of GC on HSPs expression. Dexamethasone was reported to induce the expression of heat shock protein 72 in human cardiac myocytes in vitro.[70]
The effects of BRMs
BRMs are used in the management of asthma that is uncontrolled at medium to high doses of inhaled corticosteroids and long-acting beta-agonist.[71] The commonly used BRMs are cytokine and cytokine receptor antagonists.[72,73] Only a small fraction of patients with asthma is treated using these drugs, though the guidelines on their use are increasingly being modified. The success of the therapy depends on the correct match between the biologic response modifier and the asthma endotype.[74-77] The drugs are currently used for the management of severe atopic and eosinophilic endotypes of asthma. It is proposed that these drugs modify the manifestation of COVID-19 among asthma patient [Table 3]. The most commonly used BRMs in the management of asthma are as follows: Omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab.
| Biologic | MOA | Indications | Possible role in COVID-19 |
|---|---|---|---|
| Omalizumab | Recombinant humanized IgG anti-IgE | Used to treat moderate-to-severe atopic asthma | Prevents the release of early and late phase mediators including allergic inflammatory cytokines from respiratory mast cells,[97]which may contribute to the pathogenesis of multisystem inflammatory syndrome in COVID-19[98,99] |
| Mepolizumab | Humanized IgG1 anti-IL-5 | Used for the management of severe eosinophilic asthma | Lower eosinophil count. Eosinophilia is a good prognostic marker of COVID-19.[73]Countering IL-4 and 5 signaling may favor Th1 response and perhaps quick viral clearance.[100] |
| Reslizumab | Humanized IgG1 anti-IL-5 | ||
| Benralizumab | Anti-IL-5Rα | ||
| Dupilumab | Anti-IL-4Rα |
Omalizumab was approved in 2003 for the management of severe to moderate asthma as an add on therapy,[74] urticarial, and other mast cell disorders. Several case reports on the possible role of omalizumab in reducing the severity of COVID-19 have been reported. Eksu et al., 2021,[78] and Abduelmula et al., 2021,[79] reported no hike in COVID-19 cases among patients with asthma and patients with chronic urticaria on omalizumab. Mepolizumab and reslizumab are anti-IL-5 drugs, they are used in the management of severe eosinophilic asthma.[72] They bind to free IL-5 thus making it unavailable for binding to eosinophils through IL-5R [Table 3]. Benralizumab is an anti-IL-5Rα antagonist which prevents downstream signal transduction by the IL-5R receptor.[72] IL-5 stimulates eosinopoiesis and promotes survival and recruitment of eosinophils to airways.[73] Benralizumab was used in two patients, one elderly (66 years) who did not develop severe COVID-19.[80] Eger et al., 2020,[81] reported more cases of severe COVID-19 among asthma patients treated mepolizumab, reslizumab, and benralizumab compared to the general Dutch population. Of these, nine patients were infected and six of them were hospitalized due to COVID-19-associated respiratory symptoms. However, most of these patients have other comorbidities such as obesity and diabetes.
CONCLUSION
From the available studies, asthma is generally not much a comorbidity of COVID-19 despite the presence of a favorable environment of inflamed and hyper-responsive airways. Reduced expression of the ACE2 receptor and/or expression of a low affinity isotype, the possible effects of inhaled corticosteroids and BRMs are proposed to form the bases for this incongruity. However, these may not be exhaustive, as more information on the intricate interaction between SARSCoV-2 infection of the airways and asthma disease becomes available, our understanding of this complex association will be better.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Global Strategy for Asthma Management and Prevention. 2022. Available from: https://www.ginasthma.org [Last accessed on 2022 Dec 09]
- [Google Scholar]
- Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246.
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2: Repurposed drugs and novel therapeutic approaches-insights into chemical structure-biological activity and toxicological screening. J Clin Med. 2020;9:2084.
- [CrossRef] [PubMed] [Google Scholar]
- Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372.
- [CrossRef] [PubMed] [Google Scholar]
- Intersubject variation in ACE2 protein expression in human airway epithelia and its relationship to severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2021;224:1357-61.
- [CrossRef] [PubMed] [Google Scholar]
- Centers for disease control and prevention, coronavirus disease 2019 (covid19), people with moderate to severe asthma. 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extraprecautions/asthma [Last accessed on 2022 Mar 10]
- [Google Scholar]
- Asthma and COVID-19: Emphasis on adequate asthma control. Can Respir J. 2021;2021:9621572.
- [CrossRef] [PubMed] [Google Scholar]
- Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy. 2020;27:14517.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of 138 hospitalized patients with 2019 novel corona virus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061-9.
- [CrossRef] [PubMed] [Google Scholar]
- Do chronic respiratory diseases or their treatment affect the risk of SARSCoV-2 infection? Lancet Respir Med. 2020;8:436-8.
- [CrossRef] [PubMed] [Google Scholar]
- Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826-34.
- [CrossRef] [PubMed] [Google Scholar]
- The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13:38-43.
- [CrossRef] [PubMed] [Google Scholar]
- Host and viral determinants of respiratory syncytial virus-induced airway mucus. Ann Am Thorac Soc. 2018;15:S205-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evolving concepts in how viruses impact asthma: A work group report of the microbes in allergy committee of the American academy of allergy, asthma & immunology. J Allergy Clin Immunol. 2020;145:1332-44.
- [CrossRef] [PubMed] [Google Scholar]
- Human rhinovirus diseases--epidemiology, treatment and prevention. Med Monatsschr Pharm. 2014;37:44-53.
- [Google Scholar]
- Rhinovirus-induced alterations on peripheral blood mononuclear cell phenotype and costimulatory molecule expression in normal and atopic asthmatic subjects. Clin Exp Allergy. 2002;32:537-42.
- [CrossRef] [PubMed] [Google Scholar]
- Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937-47.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. Am J Respir Crit Care Med. 2003;168:1091-4.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-7.
- [CrossRef] [PubMed] [Google Scholar]
- Does COVID19 infect the brain? If so, smokers might be at a higher risk. Mol Pharmacol. 2020;97:351-3.
- [CrossRef] [PubMed] [Google Scholar]
- Expression pattern of the SARS-CoV-2 entry genes ACE2 and TMPRSS2 in the respiratory tract. Viruses. 2020;12:1174.
- [CrossRef] [PubMed] [Google Scholar]
- Heterogeneous expression of the SARSCoronavirus-2 receptor ACE2 in the human respiratory tract. bioRxiv. 2020;3
- [CrossRef] [Google Scholar]
- ACE2 cell biology, regulation, and physiological functions In: Unger T, Ulrike M, Steckelings UM, dos Santos R, eds. The Protective Arm of the Renin Angiotensin System (RAS): Functional Aspects and Therapeutic Implications. Cambridge: Academic Press; 2015. p. :185-9.
- [CrossRef] [PubMed] [Google Scholar]
- Angiotensin converting enzyme 2-Angiotensin 1-7/1-9 system: Novel promising targets for heart failure treatment. Fundam Clin Pharmacol. 2018;32:14-25.
- [CrossRef] [PubMed] [Google Scholar]
- Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260-3.
- [CrossRef] [PubMed] [Google Scholar]
- Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422.
- [CrossRef] [PubMed] [Google Scholar]
- The immunology of asthma: Asthma phenotypes and their implications for personalized treatment. Ann Allergy Asthma Immunol. 2017;117:108-14.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56:219-33.
- [CrossRef] [PubMed] [Google Scholar]
- Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80-8.e8.
- [CrossRef] [PubMed] [Google Scholar]
- IL-13-induced proliferation of airway epithelial cells: Mediation by intracellular growth factor mobilization and ADAM17. Respir Res. 2007;8:51.
- [CrossRef] [PubMed] [Google Scholar]
- Control of ADAM17 activity by regulation of its cellular localisation. Sci Rep. 2016;6:35067.
- [CrossRef] [PubMed] [Google Scholar]
- Epigenetic regulation of ACE2, the receptor of the SARS-CoV-2 virus. Genome. 2021;64:386-99.
- [CrossRef] [PubMed] [Google Scholar]
- Eosinopenia and COVID-19. J Am Osteopath Assoc. 2020;120:504-8.
- [CrossRef] [PubMed] [Google Scholar]
- Immunology of COVID-19: Current state of the science. Immunity. 2020;52:910-41.
- [CrossRef] [PubMed] [Google Scholar]
- A novel ACE2 isoform is expressed in human respiratory epithelia and is upregulated in response to interferons and RNA respiratory virus infection. Nat Genet. 2021;53:205-14.
- [CrossRef] [PubMed] [Google Scholar]
- Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014;14:203-7.
- [Google Scholar]
- The anti inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;355:2-13.
- [CrossRef] [PubMed] [Google Scholar]
- Review: How glucocorticoids affect the neutrophil life. Int J Mol Sci. 2018;19:4090.
- [CrossRef] [PubMed] [Google Scholar]
- Hematological profiles of Nigerian patients with asthma on inhaled corticosteroids. Ibom Med J. 2021;14:455-60.
- [Google Scholar]
- Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-9.
- [CrossRef] [PubMed] [Google Scholar]
- SARS: Systematic review of treatment effects. PLoS Med. 2006;9:e343.
- [CrossRef] [PubMed] [Google Scholar]
- Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-67.
- [CrossRef] [PubMed] [Google Scholar]
- Inhaled corticosteroids and COVID-19: A systematic review and clinical perspective. Eur Respir J. 2020;55:2001009.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: A national, multicentre prospective cohort study using the ISARIC WHO clinical characterisation protocol UK. Lancet Respir Med. 2021;9:699-711.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-6.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of COVID-19 on patients with asthma. Eur Respir J. 2021;57:2003142.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: Nationwide real-world evidence. J Allergy Clin Immunol. 2021;148:361-7.e13.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of novel corona virus cases in tertiary hospitals in Hubei Province. Chinese Med J. 2020;133:1025-31.
- [CrossRef] [PubMed] [Google Scholar]
- Surviving sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854-87.
- [CrossRef] [PubMed] [Google Scholar]
- Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704.
- [CrossRef] [PubMed] [Google Scholar]
- The Inhaled Corticosteroid Ciclesonide Blocks Coronavirus RNA Replication by Targeting Viral NSP15, Vol, 2020 bioRxiv. 2020 Available from: https://www.biorxiv.org/content/10.1101/2020.03.11.987016v1%0
- [CrossRef] [Google Scholar]
- Antiviral effect of budesonide against SARSCoV-2. Viruses. 2021;13:1411.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: An observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106-20.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical-pathological correlation of the pathophysiology and mechanism of action of COVID-19-a primer for clinicians. Curr Allergy Asthma Rep. 2021;21:38.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 as an immune complex hypersensitivity in antigen excess conditions: Theoretical pathogenetic process and suggestions for potential therapeutic interventions. Front Immunol. 2020;11:566000.
- [CrossRef] [PubMed] [Google Scholar]
- Robbins and Cotran Pathologic Basis of Disease (9th ed). Philadelphia, PA: Elsevier Inc; 2015.
- [Google Scholar]
- Molecular and clinical pharmacology of intranasal corticosteroids: Clinical and therapeutic implications. Allergy. 2008;63:1292-300.
- [CrossRef] [PubMed] [Google Scholar]
- Glucocorticoids : Mechanisms of action and anti-inflammatory potential in asthma. Mediators Inflamm. 1998;7:229-37.
- [CrossRef] [PubMed] [Google Scholar]
- New insights into the anti-inflammatory mechanisms of glucocorticoids: An emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology. 2013;154:993-1007.
- [CrossRef] [PubMed] [Google Scholar]
- New twists in gene regulation by glucocorticoid receptor: Is DNA binding dispensable? Cell. 1998;93:487-90.
- [CrossRef] [PubMed] [Google Scholar]
- Crosstalk between the glucocorticoid receptor and other transcription factors: Molecular aspect. Mol Cell Endocrinol. 2007;275:13-29.
- [CrossRef] [PubMed] [Google Scholar]
- Physiologic and pharmacologic effects of corticosteroids In: Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, eds. Holland Frey Cancer Medicine (6th ed). Hamilton, ON: BC Decker; 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK13780 [Last accessed on 2022 Apr 19]
- [Google Scholar]
- A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020;9:1267.
- [CrossRef] [PubMed] [Google Scholar]
- Role of heat shock proteins (HSP70 and HSP90) in viral infection. Int J Mol Sci. 2021;22:9366.
- [CrossRef] [PubMed] [Google Scholar]
- Human coronavirus dependency on host heat shock protein 90 reveals an antiviral target. Emerg Microbes Infect. 2020;9:2663-72.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of cortisol on heat shock protein 70 levels in two fish species. Gen Comp Endocrinol. 2001;124:97-105.
- [CrossRef] [PubMed] [Google Scholar]
- Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol Endocrinol. 1996;10:705-12.
- [CrossRef] [PubMed] [Google Scholar]
- Glucocorticoid receptors in bronchial epithelial cells in asthma. Am J Respir Crit Care Med. 1998;158:963-70.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of heat-shock Protein 90 in glucocorticoid-sensitive and-resistant childhood acute lymphoblastic leukaemia. Leukemia. 2003;17:1551-6.
- [CrossRef] [PubMed] [Google Scholar]
- Heat shock factor 1, streoid hormones and regulation of heat shock proteins in the heart. Am J Physiol Heart Circ Physiol. 2001;280:H455-64.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review on the use of Omalizumab for treatment of asthmatic children and adolcents. Pediatr Allergy Immunol. 2015;26:551-6.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-interleukin-5 therapy in severe asthma. Eur Respir Rev. 2013;22:251-7.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834.
- [CrossRef] [PubMed] [Google Scholar]
- Omalizumab for asthma in adults and children. Chochrane Database Syst Rev. 2014;1:CD003559.
- [CrossRef] [Google Scholar]
- Mepolizumab for severe eosinophilic asthma (DREAM): A multiceneter double blind placebo controlled trial. Lancet. 2012;380:651-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicenter, parallel, double blind, randomised, placebo controlled, Phase III trials. Lancet Respir Med. 2015;3:355-66.
- [CrossRef] [PubMed] [Google Scholar]
- Oral glucocorticoid sparing effect of Benralizumab in severe asthma. N Engl J Med. 2017;376:2448-58.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 in patients with severe asthma using biological agents. Tuberk Toraks. 2021;69:433-6.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of COVID-19 in patients with chronic idiopathic urticaria and asthma on Omalizumab: A multicentre retrospective cohort study. J Cutan Med Surg. 2022;26:319-20.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 in two severe asthmatics receiving benralizumab: Busting the eosinophilia myth. ERJ Open Res. 2020;6:457-2020.
- [CrossRef] [PubMed] [Google Scholar]
- Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir Med. 2021;177:106287.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic associations with viral respiratory illnesses and asthma control in children. Clin Exp Allergy. 2016;46:112-24.
- [CrossRef] [PubMed] [Google Scholar]
- Polymorphisms of STAT-6, STAT-4 and IFN-c genes and the risk of asthma in Chinese population. J Respir Med. 2007;101:1977-81.
- [CrossRef] [PubMed] [Google Scholar]
- Severe asthma with blood hypereosinophilia associated with JAK2 V617F mutation: A case series. Eur Respir J. 2019;53:1802248.
- [CrossRef] [PubMed] [Google Scholar]
- The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of the myxovirus resistance A (MxA) gene-123C>a single-nucleotide polymorphism in suppressed interferon beta induction of severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2010;201:1899-908.
- [CrossRef] [PubMed] [Google Scholar]
- Polymorphisms of interferon-inducible genes OAS-1 and MxA associated with SARS in the Vietnamese population. Biochem Biophys Res Commun. 2020;329:1234-9.
- [CrossRef] [PubMed] [Google Scholar]
- Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J Virol. 2012;86:13445-55.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D levels and Vitamin D receptor gene polymorphisms in asthmatic children: A case-control study. Paediatr Allergy Immunol. 2015;26:545-50.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D receptor ApaI a allele is associated with better childhood asthma control and improvement in ability for daily activities. OMICS. 2014;18:673-81.
- [CrossRef] [PubMed] [Google Scholar]
- The Vitamin D receptor and T cell function. Front Immunol. 2013;4:148.
- [CrossRef] [PubMed] [Google Scholar]
- Ube2D3 and Ube2N are essential for RIG-I-mediated MAVS aggregation in antiviral innate immunity. Nat Commun. 2017;8:15138.
- [CrossRef] [PubMed] [Google Scholar]
- The role of host eIF2? in viral infection. Virol J. 2020;17:112.
- [CrossRef] [PubMed] [Google Scholar]
- Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052-9.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;46:110-8.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of comorbid asthma on severity of coronavirus disease (COVID-19) Sci Rep. 2020;10:21805.
- [CrossRef] [PubMed] [Google Scholar]
- Omalizumab in asthma: An update on recent developments. J Allergy Clin Immunol Pract. 2014;2:525-36.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564-81.
- [CrossRef] [PubMed] [Google Scholar]
- Omalizumab and COVID19 treatment: Could it help? Dermatol Ther. 2020;33:e13792.
- [CrossRef] [Google Scholar]
- Impact of anti-Type 2 inflammation biologic therapy on COVID-19 clinical course and outcome. J Inflamm Res. 2021;14:6845-53.
- [CrossRef] [PubMed] [Google Scholar]








