Translate this page into:
Outreach camps for awareness, screening of diabetic retinopathy among the rural population – Highlighting the need of hour

*Corresponding author: Rajwinder Kaur, Department of Ophthalmology, Adesh Institute of Medical Sciences and Research, Bathinda, Punjab, India. drrajwinder79@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chopra K, Kaur R, Sidhu TK. Outreach camps for awareness, screening of diabetic retinopathy among the rural population – Highlighting the need of hour. Adesh Univ J Med Sci Res. 2024;6:22-7. doi: 10.25259/AUJMSR_34_2024
Abstract
Objective
To examine the effectiveness of Outreach Camps Diabetic Retinopathy (DR) screening and spreading awareness within rural communities.
Material and Methods
This was an analytical study and cross sectional study in rural area of Punjab. Diabetic patients (age 18 and above) attending eye camps who gave consent were enlisted in the study. Participants were screened, educated, and surveyed. A questionnaire-based study (April 2021–March 2022) was aimed to capture the observations of the prevailing knowledge and awareness regarding diabetes among patients. Patients with DR changes were referred and managed at a tertiary hospital. The data were analyzed to establish the effectiveness of utilization of the camps for DR screening and associated factors.
Results
Of the 340 participants (680 eyes) examined, 315 eyes were included in the study. Eighty-eight patients (27.94%) had findings of DR. A significant correlation was found between the presence of DR and the duration of diabetes, age, education, and economic status. The study also highlights that compliance with hypoglycemic drugs was high (82.6%), but regular monitoring was comparatively poor (57.4%). Vision-related complaints were the most common among diabetic patients (82.2%), yet only 28.8% underwent annual eye check-ups. Awareness of DR was low (17.7%); knowledge was primarily shared by doctors, who served as the main source of information.
Conclusion
This study highlights the crucial role of outreach camps in screening and awareness of diabetic retinopathy. People belonging to low socio-economic status and low literacy rate are often neglected. Door step healthcare services and timely intervention can prevent vision threatening diabetic retinopathy in this rural population of developing countries like India.
Keywords
Awareness
Diabetic retinopathy
Outreach camps
Screening
INTRODUCTION
Diabetes mellitus (DM) is an upsurging epidemic worldwide. Approximately 77 million people in India currently have diabetes, and by 2045, that figure is expected to increase to 125 million. In India, as per literature, every one in five adults has diabetes.[1] Diabetic retinopathy (DR) is the main contributor of preventable blindness affecting around 2.5 million people globally.[2] As the disease progresses, intraretinal microvascular abnormalities and retinal hypoxia cause the release of vascular endothelial growth factor (VEGF), and leads to the formation of new vessels at the optic disc and elsewhere on the retina which can result in sudden vision loss due to vitreous hemorrhage.[3] Chronic hyperglycemia affects the inner blood-retinal barrier, resulting in diabetic macular edema and central vision loss.[4,5]
Seventy-two percent of the Indian population resides in rural regions, yet the majority of ophthalmologists are located in urban areas. Consequently, rural residents may struggle to access professional eye care. Highlighting the awareness of health education related to DM with limited socioeconomic status and educational backgrounds could help bridge this gap.[6 -8]
Vision-threatening DR (VTDR) places significant financial strain on families, communities, and healthcare systems, necessitating frequent injections for treating diabetic macular edema and expensive surgeries like pars plana vitrectomy for vitreous hemorrhage and tractional retinal detachment. Our study aims to increase awareness regarding annual screening for DR to prevent irreversible sight-threatening complications among diabetic patients.
MATERIAL AND METHODS
This research study was conducted as an analytical cross-sectional study (April 2021–March 2022) at tertiary hospital in rural setting. The targeted population was the diabetic population attending the outreach camps conducted by the department of community medicine and department of ophthalmology jointly. Authorization was granted by the Institutional Research and Ethics Committee. The sample size was calculated taking into account a total prevalence rate of 33% and an acceptable margin of error of 5%; the sample size was determined using the formula: n= (Zα/2)2 PQ/D2. In this formula, Zα/2 = 1.96, P represents the prevalence rate (33%), D is the absolute margin of error (5%), and Q = (100 - P) = 67%. The sample size (minimum) was computed as follows: (1.96)2 × (33) × (67)/(5)2 = 8490/25 = 339.6, rounded to approximately 340 total patients.[6] After informed consent, all patients aged 18 years or older were subjected to awareness all. Participants with congenital eye disease, eye trauma from the past, and previously established cognitive impairments that could hinder their ability to complete the survey or adhere to instructions or with a visually impairing cataract or corneal opacity were excluded from the study.
Four outreach camps were conducted in rural areas, preceded by pamphlet distribution advertising free eye check-ups. The optimal cutoff values for forecasting type 2 diabetes were 101 mg/dL for fasting plasma glucose and 124 mg/dL for 2-h postprandial levels, which were screened in the camp[9] [Figure 1]. Sociodemographic data were collected through expert-validated interviewer-administered questionnaires in the local language. The surveys evaluated knowledge, perceptions, and behaviors related to diabetes and retinopathy, providing insights to shape information, education, and communication strategies which were prepared by the Department of Community Medicine and Ophthalmology jointly.[8] The economic status was assessed according to PG Prasad income scale.[10] Hypertension and other systemic illnesses were noted. Education on diabetes and its complications was provided through activities including counseling using audio-visual aids and role-plays [Figure 2]. Discussions emphasized early screening benefits, with patients encouraged to spread knowledge to their social circles. The role plays performed by field staff and interns highlighted retinopathy precautions and the benefits of timely follow-ups like early screening and timely intervention of DR.
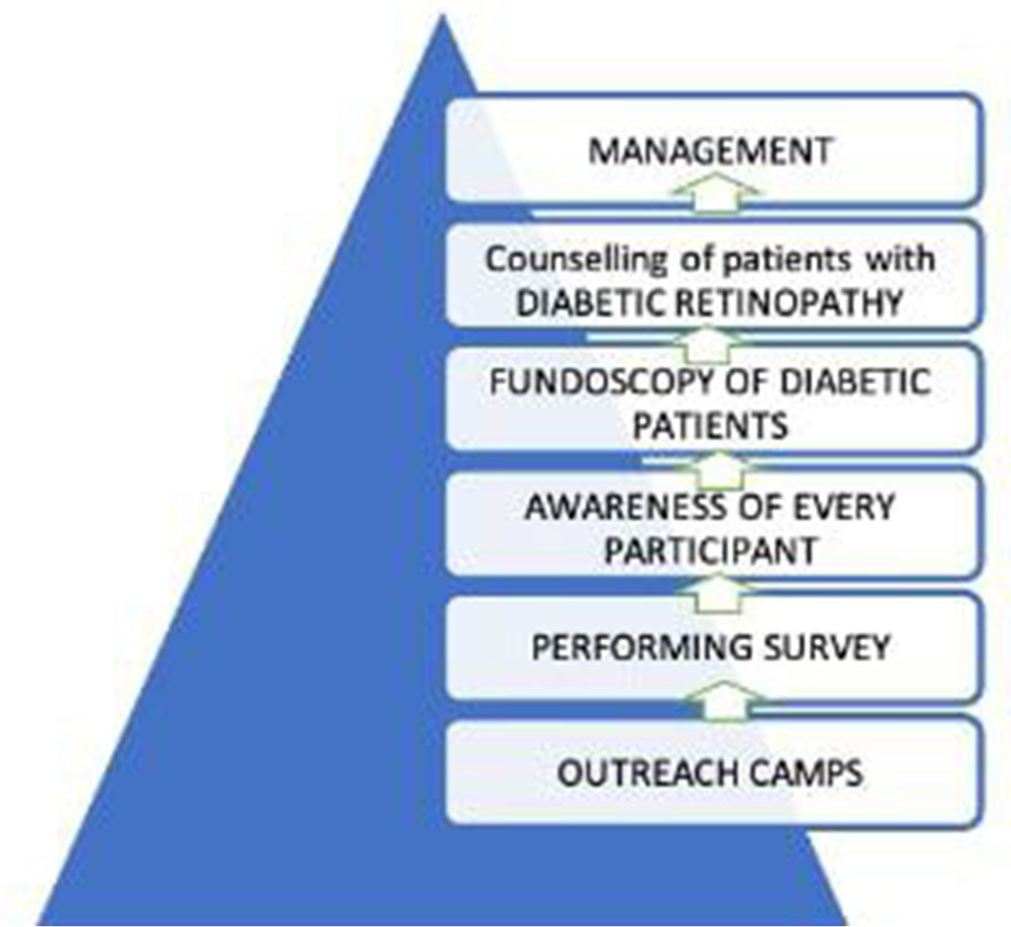
- Protocol to screen.
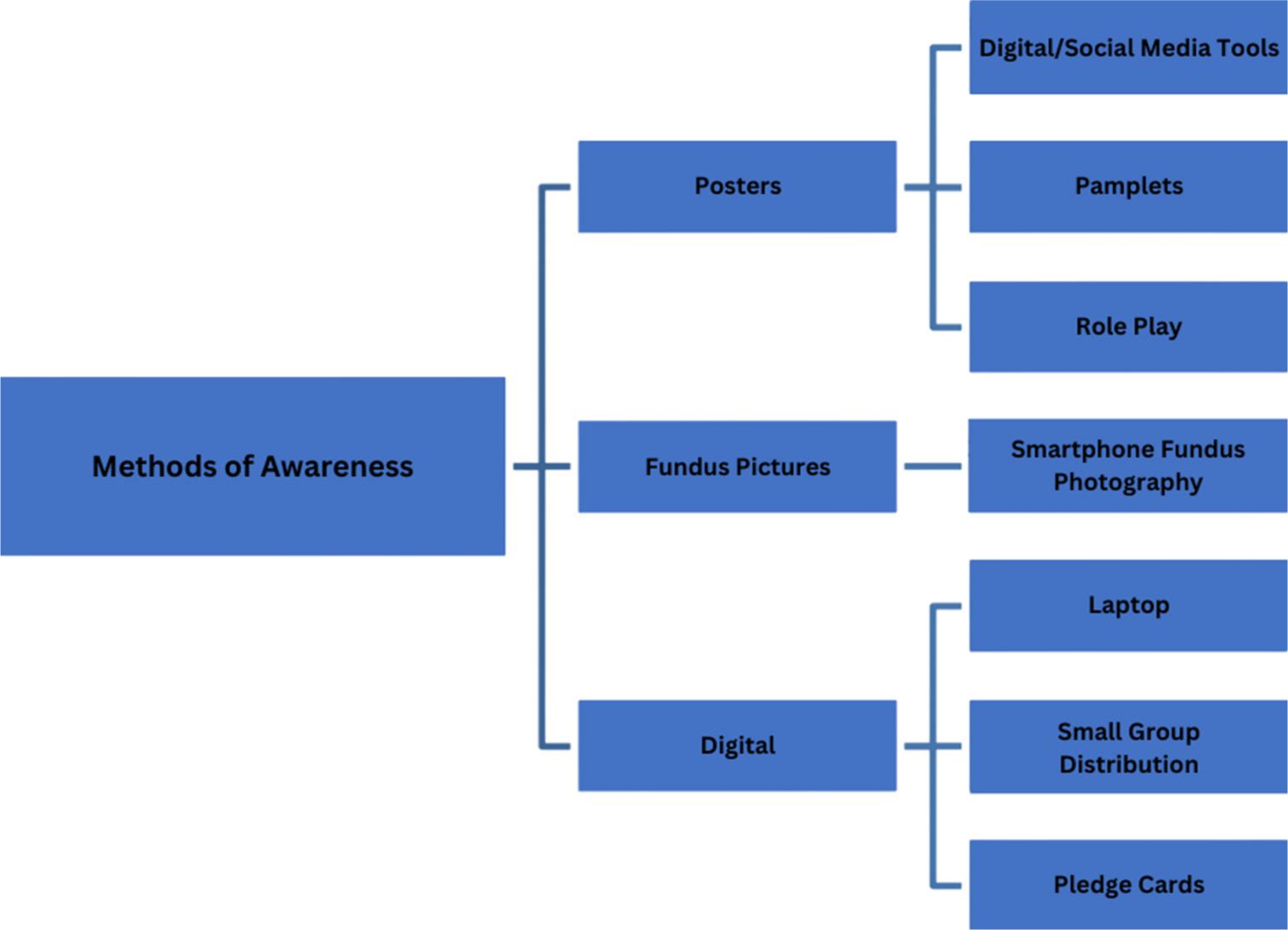
- Methods of awareness.
The ocular examination was done with the help of a torch. The best-corrected visual acuity for each eye was assessed using the Snellen chart. During the camp, a qualified ophthalmologist examined diabetic patients’ fundus at the camp after dilating with tropicamide and phenylephrine eye drops with the help of an indirect ophthalmoscope. The classification of DR, including DME, was conducted according to the early treatment DR study guidelines.[1,11] Criteria for grading of clinically significant macular edema (CSME) was scored as Grade 0 (normal): no visible exudates; Grade 1: No CSME: The shortest distance between macula and exudates >one disc diameter (DD) and Grade 2; CSME: The shortest distance between macula and hard exudates </= one DD.[11] Each eye was graded separately. Retinal images of patients with DR were taken using smartphones and a non-contact 20D lens (Volk Optical Inc., Mentor, OH, USA). These images were shown at the campsite event for counseling and management discussions. All patients with DR changes were brought to the tertiary care facility for detailed evaluation and management, blood work up to know the status of diabetes, physician consultation for diabetic control and management of ocular complications of DR.
Participant information was documented in Microsoft Excel and analyzed. The listed patients from camps were later on followed up through telephonic reminders. Quantitative data were displayed as mean and median values, while qualitative data were expressed as percentages and proportions, evaluated using the Chi-square test, with significance established at P < 0.05.
RESULTS
A total of 340 patients underwent screening and were included in the study.The average age of patients was 55.58±13 years.
Table 1 depicts the demographic characteristics of the study population expressed as percentages. In our study, various systemic illnesses were observed among diabetic patients. The prevalence rates were as follows: Hypertension (13.6%), ascertained using already available health records (4.76%), diabetic foot (4.76%), smoking or drug use (4.76%), and heart problems (5.07%). Interestingly, a majority of patients (55.55%) had no prior history of systemic illness.
| Variable | Diabetic retinopathy present | Diabetic retinopathy absent | Total (%) | P-value |
|---|---|---|---|---|
| Number of patients | 88 | 227 | 315 | |
| Gender | ||||
| Male | 50 | 96 | 146 (46.34%) | 0.02 Significant P<0.05 |
| Female | 38 | 131 | 169 (53.65%) | |
| Age (years) | ||||
| 18–40 | 5 | 47 | 52 | 0.006596 Significant P<0.05 |
| 41–60 | 47 | 102 | 149 | |
| 61–80 | 36 | 71 | 107 | |
| >80 | 0 | 7 | 7 | |
| Duration of diabetes (years) | ||||
| 0–10 | 57 | 138 | 195 | 0.039305 Significant P<0.05 |
| 11–20 | 26 | 108 | 134 | |
| 21–40 | 5 | 31 | 36 | |
| Education | ||||
| Illiterate | 33 | 158 | 191 | 0.000001 Significant P<0.05 |
| Primary | 44 | 59 | 103 | |
| Secondary | 1 | 8 | 9 | |
| Refused to answer | 10 | 2 | 12 | |
| Economic status (BG Prasad income scale) | ||||
| Low | 51 | 184 | 235 | 0.000024 Significant P<0.05 |
| Medium | 37 | 43 | 80 |
The prevalence of DR was 27.94% (88 patients). The remaining 227 patients (72.94%) had no evidence of DR. Table 2 shows the ocular features of eyes affected by DR. The severity of DR suggested the presence of proliferative DR (PDR) with vitreous hemorrhage (n = 34 eyes, 19.31%) and moderate non-PDR (NPDR) (n = 31 eyes, 17.61%) to be the most common, followed by severe NPDR (n = 16 eyes, 9.09%) and mild NPDR (n = 11 eyes, 6.25%), while PDR with pre-retinal bleed and very severe NPDR was found in 3 eyes each, respectively (1.7%). Out of 88 DR patients, 72 patients reported to the tertiary care hospital’s department of ophthalmology for further management. Clinically significant macular edema grading based on hard exudate distribution: Grade 1 (no CSME) was noted in 4 eyes (2.27%), while Grade 2 (CSME) was noted in 33 eyes (18.75%). About 65 patients (36.93%) with DR were managed by laser therapy, and 33 (18.75%) patients with CSME were administered with anti-vascular endothelial growth factor (VEGF) medications (Bevacizumab 1.25 mg in 0.05 mL intravitreal route) [Table 2]. Patients were asked to frequently visit as per the grading of the DR found. All diabetic patients with no DR were instructed to maintain strict blood sugar control and schedule regular annual dilated fundus examinations by ophthalmologists. The surveys evaluation regarding knowledge, perceptions, and behaviors related to diabetes and retinopathy is shown expressed as percentages in Table 3.
| Ocular characteristics (88 patients) | n=176 eyes | |
|---|---|---|
| BCVA (in LogMar) | Right eye | Left eye |
| <0.6 | 72 | 72 |
| 0.6–1 | 12 | 13 |
| >1 | 4 | 3 |
| Classification | ||
| Mild NPDR | 11 (6.25%) | |
| Moderate NPDR | 31 (17.61%) | |
| Severe NPDR | 16 (9.09%) | |
| Very severe NPDR | 6 (3.4%) | |
| Early PDR | 34 (19.31`%) | |
| PDR – Pre-retinal bleed/vitreous hemorrhage | 6 (3.4%) | |
| Tractional retinal detachment | 3 (1.7%) | |
| Diabetic macular edema | ||
| No macular edema (Grade 0) | 51 (28.97%) | |
| Non CSME (Grade 1) | 4 (2.27%) | |
| CSME (Grade 2) | 33 (18.75%) | |
| Management | (n=72×2 = 144 eyes) | |
| Laser | 65 (45.13%) | |
| Anti VEGF drugs | 33 (22.91%) | |
| Advised strict control | 46 (31.94%) | |
| Other ocular problems | No. of patients | |
| Cataract | 25 | |
| Glaucoma | 5 | |
| Hypertensive retinopathy | 1 | |
| Grade 2 pterygium | 2 | |
| Dry eyes | 5 | |
BCVA: Best-corrected visual acuity, NPDR: Non-proliferative diabetic retinopathy, PDR: Proliferative diabetic retinopathy, CSME: Clinically significant macular edema, VEGF: Vascular endothelial growth factor
| Awareness among participants |
| Need for annual eye check ups-28.8% |
| Diabetic ocular complications-72.3% |
| Need for having controlled RBS-57.1% |
| Sudden loss of vision due to diabetes-17.7% |
| Barriers reported |
| Lack of knowledge and lack of transport services->80% |
| Self Management practices |
| -Regular monitoring of blood sugar levels-57.4% |
| Compliance /Adherence to treatment -82.6% |
RBS: Random blood sugar
DISCUSSION
In our study of 340 diabetics, 27.94% had DR. Severity varied, with PDR (19.31%), moderate NPDR (17.61%), severe NPDR (9.09%), mild NPDR (6.25%), and PDR with preretinal bleed/very severe NPDR at 1.7% and CSME (18.75%). A similar study in Central India in tertiary care hospitals found DR in 42.5% of patients, comprising 29.41% with mild NPDR, 41.18% with moderate NPDR, and 29.41% with diabetic maculopathy.[12] Our findings closely align with a North Indian hospital study (33.1% DR positive)[13] and a South Indian study (21.7%).[14] In Malaysia, community-based screening revealed a 14.9% prevalence, while in Pakistan, it ranged from 19% to 26%.[15,16]
Community outreach camps allow an opportunity for identification of DR changes among the diabetic population at the early stage,and their counseling helps in spreading the awareness for early detection by regular annual screening. Kumar et al. noted that many regions do not have a solid public health strategy for managing DR. Outreach camps in rural area hold promise for enhancing early patient identification and treatment.[17] A significant Japanese study with 66,923 diabetics found DR as the most common complication of DM (23.6%).[18] In our study, for CSME – 18.75% (33 patients) were managed using anti-VEGF drugs, while 36.93% (65 patients) with DR underwent laser therapy. Adhering to guidelines, we prioritized systemic control of glycemia and blood pressure, applying pan-retinal photocoagulation, focal, and grid photocoagulation, along with intravitreal VEGF treatment for DME.
Clinical guidelines advise diabetic patients to undergo annual eye screening.[18] Annual screening can utilize telemedicine or onsite fundus photography.[19,20] In our study, only 28.8% of diabetic patients had annual eye checkups, whereas in another study showed 55% of known diabetes patients never had eye exams.[13] In our survey, >80% reported a lack of awareness and transport services as a barrier to eye examinations. As per Backlund et al., evaluating patients in their primary healthcare settings, a more comfortable and reassuring environment reduced access obstacles.[21] Outreach centers were established to address the primary barriers to receiving care for DR in India, particularly the problems of travel and distance.[6] To bridge the gap, employing a mobile van strategy for organizing future screening campaigns of DR can enhance accessibility, boost compliance, and benefit patients.[18] The threshold of 104 mg/dL aimed to aid in the early identification of previously undiagnosed individuals during diabetes screening.[22]
Efficient diabetes control is crucial in delaying the onset of vascular issues.[22] Poor glycemic control and uncontrolled lipid profiles increased ocular complications and were linked to DR progression.[15] Our study shows that 8.8% of our participants gave a history of lipid derangement. These findings highlight the diverse health challenges faced by diabetic individuals and underscore the need for comprehensive healthcare approaches.
In a survey of 42,146 participants, diabetes was detected in 18.8% (7,910 individuals) during screening. Of these, 6133 (77.5%) had fundus changes related to DR. The results revealed a higher prevalence of DR (15.5% vs. 8.0%) and VTDR (5.3% vs. 2.4%) in undiagnosed diabetes patients compared to those diagnosed.[1] In our study, awareness about DR primarily came from outreach screening camps (47.61%) and physicians (39.3%) by ophthalmology and social and preventive medicine department along with non-governmental organisations. This underscores the importance of educating doctors, nurses, and patients to enhance coverage and improve follow-up. This emphasis shifts focus from costly tertiary treatments to prioritizing widespread primary and secondary prevention for diabetes, emphasizing self-care, awareness, and screenings.[6,13,23]
Merely 57.14% of diabetic patients in camps recognized that managing blood sugar levels helps to protect eyesight. Using posters and one on one communication was a deliberate strategy to keep diabetics informed and connected, reducing missed follow-up appointments and preserving early screening benefits . Evidence shows only a slight increase in screening rates following the second reminder.[24] Screening camps and hospital counseling sessions offer ideal chances for awareness.[18,24]
Implications
Our findings highlight the need to expand DR screening beyond hospital settings to rural outreach camps in Punjab. Efficient methods for enrollment, evaluation, and follow-up are essential for enhancing screening rates and reducing long-term complications. AI-driven teleophthalmology, employing tools such as the Make in India Retcam, enables direct communication between ophthalmologists and healthcare providers, enhancing accessibility and effectiveness in underserved areas.[25]
CONCLUSION
Initiatives like DR outreach camps offer a promising chance to reduce disease burden, especially in resource-limited settings. Utilizing posters, screening camps, and hospital counseling effectively enhances awareness and reduces vision loss related to DR.
Ethical approval
The research/study approved by the Institutional Review Board at Adesh Institute of Medical Sciences and Research, number AU/EC_2K23/333, dated 10th March, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Rajwinder Kaur and Dr.Tanvir Kaur Sidhu are on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Prevalence of diabetic retinopathy in India stratified by known and undiagnosed diabetes, urban-rural locations, and socioeconomic indices: Results from the SMART India population-based cross-sectional screening study. Lancet Glob Health. 2022;10:e1764-73.
- [CrossRef] [PubMed] [Google Scholar]
- An assessment on the awareness of diabetic retinopathy among participants attending the diabetes awareness camp in Saudi Arabia. Cureus. 2022;14:e31031.
- [CrossRef] [Google Scholar]
- Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-4.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52:2160-4.
- [CrossRef] [PubMed] [Google Scholar]
- Factors that influence the patient uptake of diabetic retinopathy screening. Ir J Med Sci. 2008;177:303-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic retinopathy in the Asia-Pacific. Asia Pac J Ophthalmol (Phila). 2018;7:3-16.
- [Google Scholar]
- Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69:2932-8.
- [CrossRef] [PubMed] [Google Scholar]
- The English national screening programme for sight-threatening diabetic retinopathy. J Med Screen. 2008;15:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Cut-off values of fasting and post-load plasma glucose and HbA1c for predicting Type 2 diabetes in community-dwelling Japanese subjects: The Hisayama Study. Diabet Med. 2012;29:99-106.
- [CrossRef] [PubMed] [Google Scholar]
- Updated BG Prasad's socioeconomic status classification for the year 2023. Indian J Community Med. 2023;48:934-6.
- [CrossRef] [PubMed] [Google Scholar]
- An automated system for the grading of diabetic maculopathy in fundus images In: International conference on neural information processing. Springer-Verlag; 2012. p. :36-43.
- [CrossRef] [Google Scholar]
- Prevalence and awareness of diabetic retinopathy in diabetic patients visiting tertiary care hospitals in central India. Cureus. 2023;15:e39414.
- [CrossRef] [Google Scholar]
- Awareness of diabetic retinopathy among diabetes mellitus patients visiting a hospital of North India. J Family Med Prim Care. 2022;11:1292-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and risk factors for diabetic retinopathy: A population-based assessment from Theni District, south India. Br J Ophthalmol. 2009;93:429-34.
- [CrossRef] [Google Scholar]
- Diabetic eye screening in Malaysia: Findings from the National Health and Morbidity Survey 2006. Singapore Med J. 2010;51:631-4.
- [Google Scholar]
- Ocular complications associated with diabetes and the risk of sustainable blindness; A real world analysis. J Pak Med Assoc. 2023;73:1453-6.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic retinopathy screening and management in India: Challenges and possible solutions. Indian J Ophthalmol. 2021;69:479-81.
- [CrossRef] [PubMed] [Google Scholar]
- Recent epidemiological status of ocular and other major complications related to diabetes mellitus in Japan. Ophthalmologica. 2020;243:404-12.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic retinopathy screening guidelines in India: All India Ophthalmological Society diabetic retinopathy task force and Vitreoretinal Society of India Consensus Statement. Indian J Ophthalmol. 2021;69:678-88.
- [CrossRef] [PubMed] [Google Scholar]
- ISPAD clinical practice consensus guidelines 2022: Microvascular and macrovascular complications in children and adolescents with diabetes. Pediatr Diabetes. 2022;23:1432-50.
- [CrossRef] [PubMed] [Google Scholar]
- Early detection of diabetic retinopathy by a mobile retinal photography service working in partnership with primary health care teams. Diabet Med. 1998;15(Suppl 3):S32-7.
- [CrossRef] [Google Scholar]
- Diagnostic accuracy of tests for type 2 diabetes and Prediabetes: A systematic review and meta-analysis. PLOS One. 2020;15:e0242415.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246-58.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of interventions to promote screening for diabetic retinopathy. Am J Prev Med. 2007;33:318-35.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic retinopathy screening using MII Ret Cam assisted smartphone-based fundus imaging. J Med Surg Public Health. 2024;2:100068.
- [CrossRef] [Google Scholar]








