Translate this page into:
Molecular insights into respiratory syncytial virus: Genotyping and characterization in pediatric community-acquired lower respiratory tract infections

*Corresponding author: Manju Singh, Centre for Interdisciplinary Biomedical Research, Adesh University, Bathinda, Punjab, India. manjuchaudhary080191@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hassan M, Singh M, Bhattal HS, Saini RG. Molecular insights into respiratory syncytial virus: Genotyping and characterization in pediatric community-acquired lower respiratory tract infections. Adesh Univ J Med Sci Res. doi: 10.25259/AUJMSR_2_2025
Abstract
Background
Respiratory syncytial virus (RSV) continues to pose a considerable global health concern, especially impacting newborns and young children with community-acquired lower respiratory tract infections (LRTIs).
Objective
This review aims to comprehensively analyze the molecular characteristics, genotyping methods, and epidemiological patterns of RSV, emphasizing its impact on pediatric populations and current developments in prevention strategies.
Methods
A comprehensive evaluation of current literature examining RSV’s structural components, genetic diversity, clinical manifestations, detection techniques, and vaccine development was conducted.
Results
RSV, classified into subtypes A and B, demonstrates significant genetic and antigenic variability, particularly in the G glycoprotein gene. The virus causes approximately 33 million cases of acute LRTIs annually in children under five, resulting in 3 million hospitalizations and 118,000 deaths globally. Advanced molecular techniques, including reverse transcription polymerase chain reaction and next-generation sequencing, have enhanced our understanding of RSV’s genetic diversity and transmission dynamics. Recent vaccine developments, particularly those targeting the prefusion F protein, show promising results in clinical trials, including maternal vaccination strategies for protecting neonates.
Conclusion
Understanding RSV’s molecular characteristics and genetic diversity is crucial for developing effective interventions. Continued surveillance, improved diagnostic techniques, and targeted vaccination approaches are crucial for the management of RSV infections and reducing their global health burden.
Keywords
Bronchiolitis
Epidemiology
Genetic diversity
Genotyping
Lower respiratory tract infections
Molecular characterization
Pediatric infections
Respiratory syncytial virus
Reverse transcription polymerase chain reaction
Vaccination
INTRODUCTION
Respiratory syncytial virus (RSV) is a pervasive pathogen with a profound impact on global health, particularly among infants and young children. As a member of the Paramyxoviridae family and the Orthopneumovirus genus, RSV exhibits a high degree of genetic and antigenic variability, allowing it to evade immune defenses and reinfect individuals throughout their lives.[1] Characterized by its single-stranded negative-sense ribonucleic acid (RNA) genome, RSV has been categorized into two major subtypes, RSV-A and RSV-B, which further diversify into numerous genotypes.[2] These genetic differences not only drive its extensive epidemiological reach but also influence therapeutic results, ranging from moderate respiratory symptoms to serious infections of the lower respiratory tract, such as bronchiolitis and pneumonia.[3]
Globally, RSV is a primary contributor to pediatric hospitalizations, accounting for millions of sudden-onset respiratory infections annually. Babies younger than 6 months are disproportionately affected due to their immature immune systems and smaller airways.[4] Despite its significant burden, RSV remains a challenging pathogen to combat due to its antigenic drift, recombination, and lack of lasting immunity.[5] Recent advancements in molecular characterization and genotyping techniques, including reverse transcription polymerase chain reaction (RT-PCR) and next-generation sequencing (NGS), have offered a valuable understanding of its genetic diversity and transmission dynamics.[6] These findings have underscored the necessity for customized public health strategies, including surveillance, vaccine development, and antiviral therapies.
This review brings together existing knowledge on RSV’s molecular biology, epidemiology, and clinical manifestations. It emphasizes the importance of genotyping in understanding strain-specific pathogenicity and guiding interventions.[7] In addition, it highlights the global implications of RSV genetic diversity for vaccine efficacy and the challenges of managing severe infections in vulnerable populations.[8] By addressing these aspects, the article aims to contribute to the growing body of research that seeks to mitigate the impact of RSV on public health.
STRUCTURAL AND MOLECULAR CHARACTERISTICS OF RSV
RSV is an enveloped virus belonging to the family Paramyxoviridae and genus Orthopneumovirus. The virion typically measures 150–300 nm and features a single-stranded RNA genome with negative-sense orientation encoding 10 genes. These genes produce 11 proteins, categorized as structural (F, G, SH, N, P, M) and non-structural (NS1, NS2).[9] The structural proteins include glycoproteins, matrix proteins, and nucleocapsid proteins, all of which play integral roles in the virus’s replication and ability to escape detection by the host’s immune system [Figure 1].
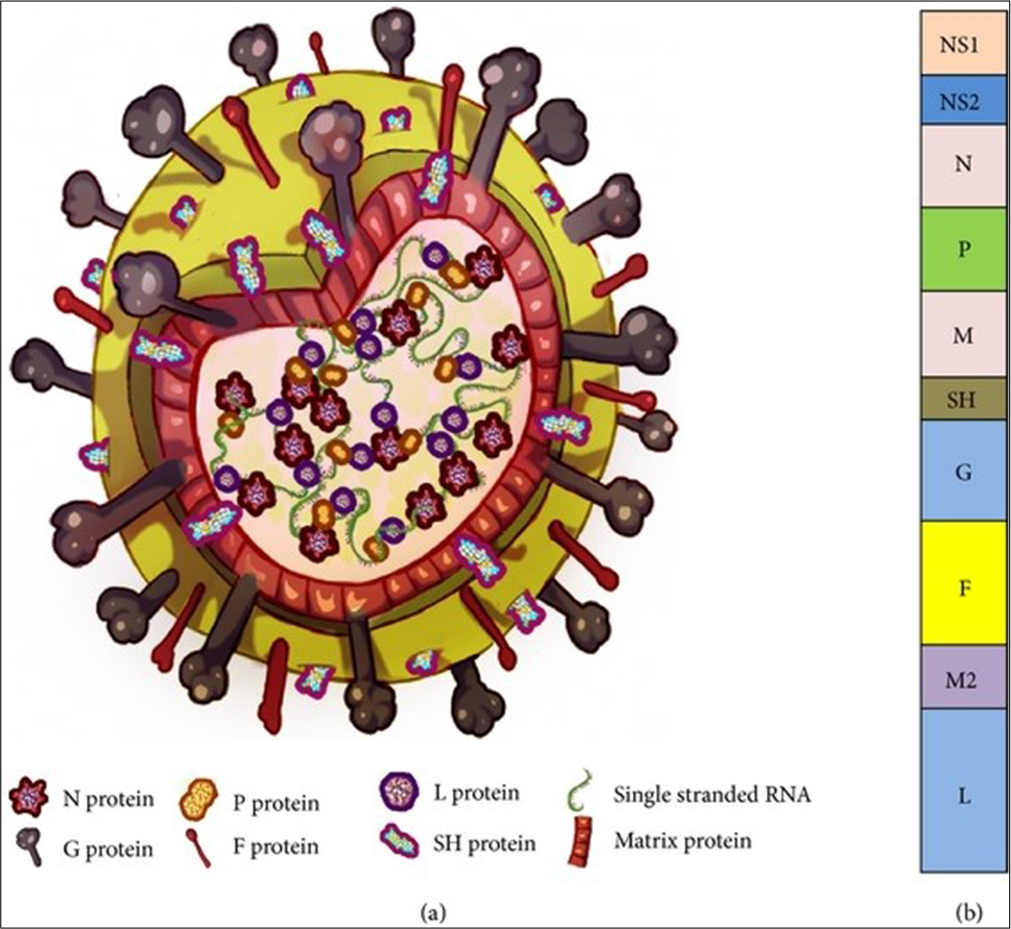
- Structure and genome organization of respiratory syncytial virus (RSV). (a) Approximately 150–300 nm RSV virion particle and (b) single-stranded negative RNA genome consisting of 10 genes (Bawage et al., 2013). G: Glycoprotein, F: Fusion protein, SH: SH protein, M: Matrix protein, N: Nucleoprotein, P: Phosphoprotein, L: “Large” protein, RNA: Ribonucleic acid
The lipid envelope of RSV contains glycoproteins such as the attachment (G) and fusion (F) proteins, which assist in the viral entry into host cells. The G protein binds to cellular receptors, including heparan sulfate proteoglycans, while the F protein mediates membrane fusion, allowing viral genetic material to enter the host cell. The small hydrophobic protein, another surface component, plays a role in ion channel formation and contributes to RSV’s pathogenicity.[10] Inside the virion, the matrix (M) protein is critical for viral assembly, while the nucleoprotein (N), phosphoprotein (P), and RNA-dependent RNA polymerase (L) form the ribonucleoprotein complex essential for replication.[11] Nonstructural proteins NS1 and NS2 inhibit the host immune response, enabling RSV to evade innate defences[12] [Table 1].
| Position within the virion | Protein | Alternative name | Function | Additional information |
|---|---|---|---|---|
| Lipid envelope (Membranespanning surface proteins) | G | Glycoprotein | Viral attachment to the ciliated cells in the host’s airways | The F and G glycoproteins are the principal surface proteins that regulate the attachment of viruses and the earliest phases of infection. In addition, F and G proteins serve as the key target for eliminating antibodies throughout the course of infection. |
| F | Fusion protein | Merging of the viral and host cell membranes; syncytium formation | ||
| SH | SH protein | Viroporin; ion channel | Engages in cell fusion, although lacks a recognized neutralizing epitope. | |
| Inner envelope face | M | Matrix protein | Assembly | Engaged in genome transcription, RNA replication, and the process ofparticle budding. |
| Ribonucleocapsid | N | Nucleoprotein | RNAbinding | |
| P | Phosphoprotein | Phosphorylation | ||
| L | “Large” protein | RNAguided RNA polymerase | ||
| M21 | Transcription processivity factor | |||
| Regulatory | M22 | Regulation of transcription/RNA replication | ||
| Nonstructural | NS1 | Involved in the circumvention of the innate immune system. | Function through suppressing apoptosis and obstructing type I Interferon signaling | |
| Nonstructural | NS2 |
SH: Small hydrophobic, RSV: Respiratory syncytial virus, RNA: Ribonucleic acid
Replication cycle
The RSV replication cycle begins with the attachment of the G protein to heparan sulfate proteoglycans on host cells, followed by membrane fusion mediated by the F protein. The viral RNA genome is transcribed and replicated in cytoplasmic inclusion bodies, where the ribonucleoprotein complex orchestrates these processes. Newly formed virions assemble at the cell membrane and bud off to infect adjacent cells, often forming syncytia that contribute to tissue damage.[13] Syncytium formation is a hallmark of RSV infection, facilitating cell-to-cell spread and immune evasion [Figure 2]. These features underscore RSV’s adaptability and persistence in host environments.
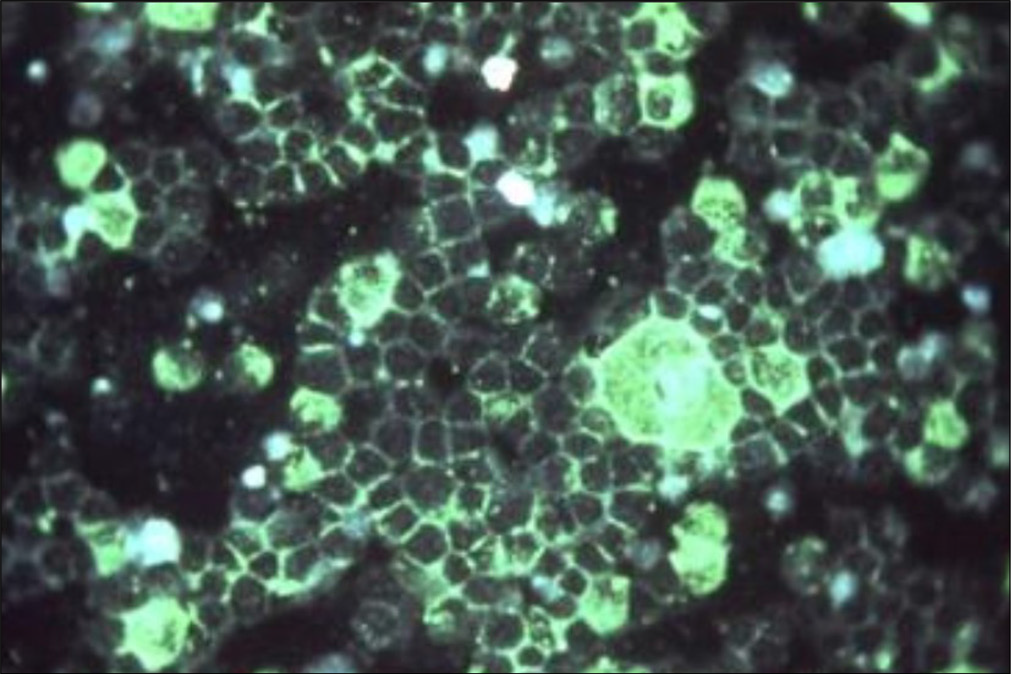
- Photomicrographic detection of respiratory syncytial virus using indirect immunofluorescence technique. (fluorescein isothiocyanate 200x to 400x) (Source: National Institute of Allergy and Infectious Diseases)
The replication process is tightly regulated, with each stage presenting potential targets for therapeutic intervention. For instance, inhibitors targeting the F protein’s conformational changes have shown promise in preclinical studies. Understanding RSV’s replication cycle is thus critical for developing antivirals and improving treatment outcomes [Figure 3].
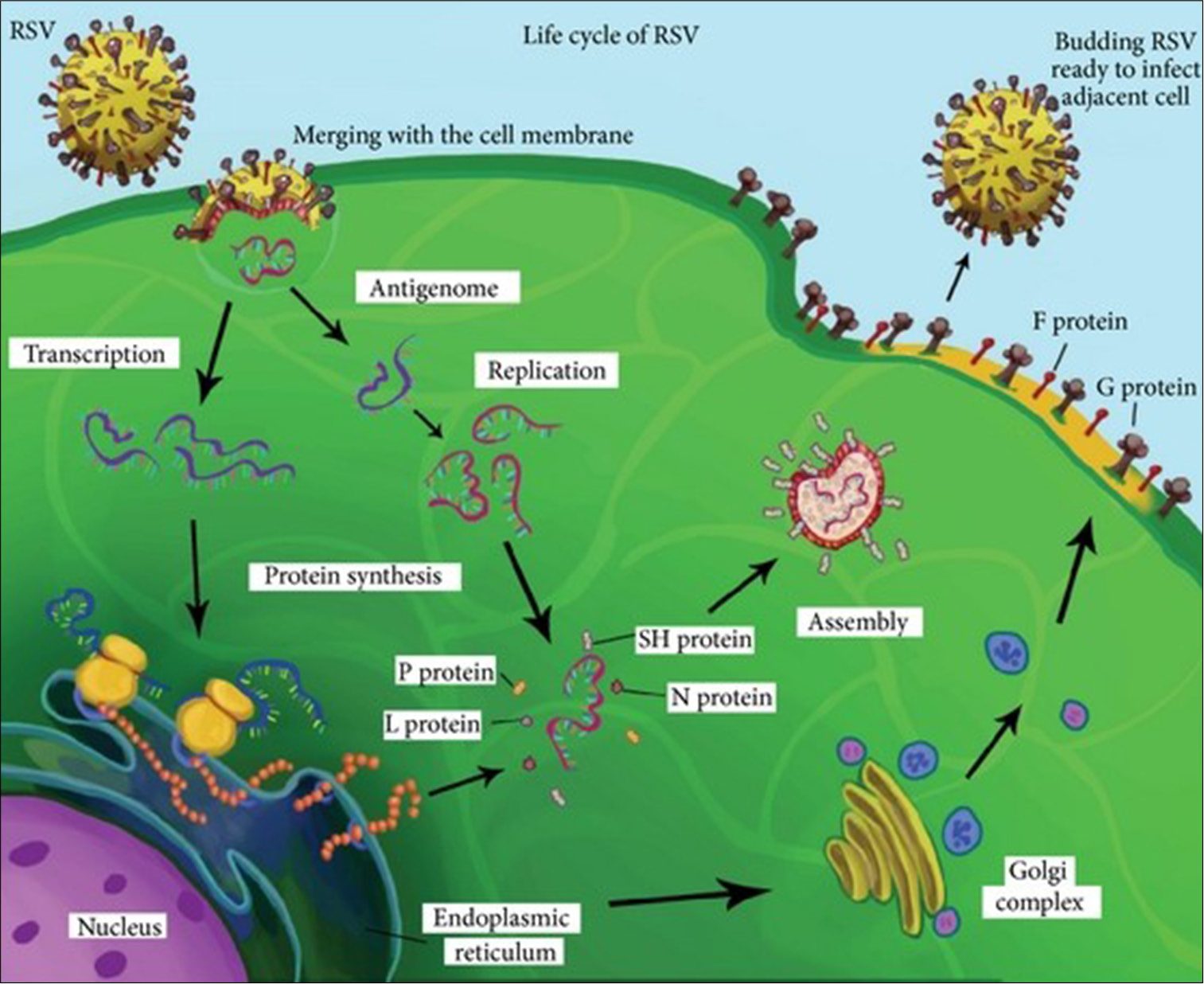
- Replication cycle of respiratory syncytial virus (RSV).
RSV SUBTYPES AND GENETIC DIVERSITY
RSV is categorized into two primary subtypes, RSV-A and RSV-B, distinguished by genetic and antigenic alterations, mainly in the G glycoprotein gene.[14] RSV-A is often linked to more severe clinical outcomes, while RSV-B tends to cause milder infections. Each subtype is further divided into genotypes: 15 for RSV-A (e.g., Ontario 1 [ON1]) and 30 for RSV-B (e.g., Buenos Aires 9 [BA9]). Notable genotypes such as ON1 (RSV-A) and BA9 (RSV-B) have demonstrated significant genetic variability and clinical impact.[15]
Implications of genetic variability
The genetic evolution of RSV involves antigenic drift and recombination, particularly within the G protein gene, complicating the advancement of vaccinations and treatment approaches. Mutations in G protein’s mucin-like domain affect antigenicity and immune evasion. The F protein, although more conserved, exhibits variability in its prefusion and postfusion conformations, influencing viral infectivity and vaccine design.[16] Genetic variety has pragmatic significance for RSV surveillance and vaccine development. For instance, ON1 Genotypes are linked to increased transmissibility and severity, necessitating genotype-specific interventions. By mapping genetic changes, researchers can anticipate shifts in RSV epidemiology and refine therapeutic strategies accordingly.
“Recent molecular analyses have revealed complex evolutionary patterns in RSV strains circulating globally. The G protein gene shows remarkable plasticity, with distinct duplication events characterizing major genotypes. The ON1 genotype of RSV-A, which emerged through a 72-nucleotide duplication in the C-terminal region of the G gene, has demonstrated enhanced transmissibility and virulence.[17] Similarly, the BA genotype of RSV-B, characterized by a 60-nucleotide duplication, has shown increased fitness in population-level studies. Comprehensive genomic analyses have identified specific mutations in the F protein that affects neutralization sensitivity and vaccine efficacy.[18]
Clinical studies correlating genotypes with disease severity have shown that certain ON1 variants are associated with increased hospitalization rates and oxygen requirements, particularly in infants under 6 months.[19] The BA9 genotype of RSV-B has demonstrated enhanced replication efficiency in primary human airway epithelial cells, though its clinical implications remain under investigation.[20] These findings have crucial implications for vaccine development and therapeutic strategies.”
EPIDEMIOLOGY OF RSV
RSV accounts for approximately 33 million cases of acute lower respiratory tract infections (LRTIs) annually among children under five, leading to 3 million hospitalizations and 118,000 deaths.[21] Infants under 6 months of age are particularly vulnerable due to immature immune systems.[22] Most deaths associated with RSV transpire in low- and middle-income countries, where healthcare access is limited.[23]
Age and seasonal patterns
RSV infection rates and severity vary by age. Infants under 6 months face the greatest risk of severity disease, including pneumonia and bronchiolitis.[24] RSV follows a seasonal pattern, peaking during winter in temperate regions and aligning with rainy seasons in tropical areas.[25] The seasonality is influenced by climate factors such as temperature and humidity, which affect viral transmission rates.[26]
Regional variations
The RSV-A: RSV-B ratio exhibits significant geographical variation. In North America and Europe, RSV-A predominates, while both subtypes co-circulate in Asia and Africa. These variations are influenced by local immunity, climate, and healthcare infrastructure.[27] For example, RSV-B is more prevalent in sub-Saharan Africa, likely due to regional differences in immune pressure and viral evolution.[28]
Regional studies have highlighted the importance of tailored public health interventions. In regions with high RSV-B prevalence, vaccination strategies may need to account for genotype-specific differences to achieve optimal protection. Surveillance data also inform resource allocation during seasonal peaks, minimizing the strain on healthcare systems.
RSV burden in India
Recent epidemiological studies from India have revealed a significant RSV disease burden among children. A comprehensive surveillance study across major Indian cities found that RSV accounts for 17–32% of hospitalizations due to acute lower respiratory infections in children under 5 years.[29] The RSV-A subtype predominates in most Indian regions, with distinct seasonal patterns coinciding with monsoon months in different geographical areas. A multi-center study across six Indian states reported significantly higher hospitalization rates (45.3/1000) in infants below 6 months compared to older children.[30] Urban areas showed higher detection rates, possibly due to better surveillance systems and healthcare access. Molecular surveillance data from 2020 to 2024 indicate the circulation of multiple genotypes, with ON1 being predominant among RSV-A strains and BA9 among RSV-B strains.[31] The burden is particularly concerning in resource-limited settings where access to intensive care facilities is limited.”
CLINICAL MANIFESTATIONS OF RSV
RSV infections range from mild upper respiratory symptoms to severe conditions like bronchiolitis and pneumonia. Younger infants, particularly those under 6 months, often experience severe symptoms, including cyanosis, tachypnea, and wheezing.[24] Among older kids and grown-ups, RSV typically causes mild cold-like symptoms but may result in serious illness in immunocompromised individuals.
Variability by host factors
Disease severity is influenced by host factors such as age, prematurity, and underlying conditions (e.g., preexisting heart defects or long-term lung conditions). Immunocompromised individuals and individuals with neuromuscular disorders are also at elevated risk.[32] Genetic predispositions, including variations in innate immune genes, may also affect RSV susceptibility and outcomes.[33]
Complications
Complications of severe RSV include apnea in neonates, secondary bacterial infections, and long-term sequelae such as recurrent wheezing and asthma in children. Studies suggest a strong correlation between severe RSV bronchiolitis and the subsequent development of chronic respiratory conditions.[34] Severe cases often necessitate hospitalization, oxygen support, and, in extreme instances, mechanical ventilation. Early recognition and administration of complications are crucial for lowering illness and death rates.
DETECTION AND GENOTYPING TECHNIQUES
RT-PCR
RT-PCR is the gold standard for RSV detection, offering high sensitivity and specificity. It targets conserved sections of the genome of RSV, including the F genes and N genes, and provides quantitative viral load data.[35] Point-of-care RTPCR assays have improved the accessibility and speed of RSV diagnosis in clinical settings.
Sequencing and phylogenetics
Advancements in sequencing, including Sanger and NGS, have enhanced the characterization of RSV genotypes. Phylogenetic analysis aids in tracing RSV evolution and guiding vaccine design. NGS, despite its high cost, provides comprehensive insights into viral diversity.[36] Phylogenetic trees constructed from G and F gene sequences reveal patterns of genotype dominance and interregional spread. Molecular tools have also facilitated real-time outbreak monitoring, enabling healthcare providers to implement timely interventions. These technologies underscore the importance of integrating diagnostics with epidemiological surveillance to mitigate RSV’s impact.
RSV genotyping is crucial to understanding its epidemiology, evolution, and clinical impact. Each molecular RSV genotyping method has pros and cons. This comparison compares the three most common genotyping methods, RTPCR, Sanger Sequencing, and NGS, based on their sensitivity, specificity, and clinical relevance[37-39] [Table 2].
| Method | Sensitivity | Specificity | Clinical application |
|---|---|---|---|
| RTPCR | High | High | Routine diagnosis; epidemiological surveillance |
| Sanger Sequencing | Moderate | High | Genotyping; research applications; less common for routine diagnosis |
| NGS | Very High | High | Research; surveillance; limited routine clinical use |
RT-PCR: Reverse transcription polymerase chain reaction, NGS: Next-generation sequencing
RSV AND VACCINE DEVELOPMENT
Challenges in vaccine development
RSV’s genetic diversity and continuous evolution present significant challenges for vaccine efficacy. Variability in the F and G proteins, principal targets for eliminating antibodies, complicates the formulation of broadly protective vaccines.[40] In addition, incomplete and short-lived immunity following natural infection poses hurdles for vaccine-induced protection.
Recent advances
Promising vaccine candidates, including Pfizer’s ABRYSVO™ and GSK’s Arexvy, have shown efficacy in elderly individuals by focusing on the prefusion of F protein. These vaccines highlight the potential for age-specific formulations.[41] Maternal vaccination strategies, aimed at transferring protective antibodies to neonates are also under investigation and have demonstrated encouraging outcomes in clinical trials [Figure 4].
- Food and Drug Administration (FDA) approves respiratory syncytial virus vaccine in pregnancy to protect infants. The Washington post (Nirappil, 2023)
“The RSV vaccine landscape has evolved significantly with recent approvals. GSK’s Arexvy (RSVPreF3) utilizes a stabilized prefusion F protein with AS01 adjuvant, demonstrating 83% efficacy against severe RSV disease in adults aged 60 and older.[42] Pfizer’s ABRYSVO, a bivalent prefusion F protein-based vaccine, has shown efficacy in both maternal immunization (82% protection against severe RSV in infants through 6 months of age) and older adult populations.[43] Moderna’s mRNA-1345, approved in late 2023, represents the first mRNA-based RSV vaccine, showing 84% efficacy against RSV-associated lower respiratory tract disease in adults 60 years and older.[44]
Several promising vaccine candidates are in advanced clinical trials. These include novel live-attenuated vaccines for pediatric populations, showing favorable safety profiles and immunogenicity in phase 2 trials.[45] Vector-based vaccines using adenovirus platforms have demonstrated robust immune responses in early-phase studies.[46] In addition, combined RSV/influenza vaccines have shown promising results in phase 2 trials, potentially offering simplified vaccination schedules for older adults.[47]
Recent advances in monoclonal antibodies have significantly expanded preventive options. Nirsevimab (Beyfortus), approved in 2023, provides single-dose protection through an infant’s first RSV season, showing 74.5% efficacy against medically attended RSV LRTIs.[48] This represents a substantial improvement over palivizumab’s monthly dosing requirement. New monoclonal antibodies in development include those targeting multiple epitopes simultaneously and formulations optimized for cost-effectiveness in resource-limited settings.[49]”
IMPLICATIONS FOR PUBLIC HEALTH
Surveillance and outbreak management
Global surveillance initiatives, such as those led by the World Health Organization, monitor RSV’s genetic diversity and seasonal trends, enabling targeted public health interventions.[50] Early detection through molecular surveillance aids in outbreak management and resource allocation. Digital tools, such as real-time epidemic tracking and predictive modeling, enhance the timeliness and precision of responses to RSV outbreaks.
Tailored vaccination strategies
Population-specific vaccination strategies, informed by regional genotype prevalence, are essential for optimizing vaccine effectiveness. Molecular surveillance informs these strategies by identifying dominant strains and guiding vaccine updates.[14] For instance, incorporating regionally prevalent genotypes into vaccine formulations could enhance their protective efficacy in high-burden areas.
CONCLUSION
RSV continues to be a substantial worldwide health issue, resulting in significant illness and death rates, particularly among infants and vulnerable populations. Despite decades of research, critical gaps persist in our understanding of the interaction of RSV with the host immune system, the mechanisms driving its genetic evolution, and its variable epidemiological patterns across different regions. These gaps hinder the development of universal vaccines and effective therapeutic strategies.
At present, advancements in molecular diagnostics and genotyping have enhanced our ability to characterize the genetic diversity of RSV and track emerging strains. Real-time surveillance initiatives have improved our understanding of seasonal trends and genotype prevalence, paving the way for region-specific interventions. This review underscores the importance of integrating such data with public health strategies to optimize resource allocation during outbreaks and guide vaccine development.
The insights presented in this review are crucial for informing future research and policy-making. By highlighting the structural and genetic complexity RSV, the article emphasizes the need for multidisciplinary collaboration to address its global burden. The ongoing development of vaccines targeting the prefusion F protein and maternal immunization strategies is promising, yet sustained investment and surveillance are essential to adapt these solutions to the virus-evolving landscape.
Looking ahead, future research must focus on filling existing gaps, including understanding host-pathogen interactions, identifying long-term sequelae of severe RSV infections, and designing vaccines that offer broader and longer-lasting protection. Furthermore, equitable access to vaccines and treatments in resource-limited settings remains a critical challenge that warrants global attention.
By bridging these research and implementation gaps, the global health community can significantly reduce the impact of RSV, moving closer to achieving sustainable solutions for this pervasive pathogen.
Acknowledgment
The authors express gratitude to the Centre for Interdisciplinary Biomedical Research (CIBR) for providing the necessary resources and support that contributed to the preparation of this manuscript.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations
Financial support and sponsorship: Nil.
References
- Pneumoviruses: Respiratory syncytial virus and human metapneumovirus In: Viral infections of humans: Epidemiology and control. New York, NY: Springer US; 2023. p. :1-53.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory syncytial virus: the influence of serotype and genotype variability on clinical course of infection. Int J Mol Sci. 2017;18:1717.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and biological consequences of respiratory syncytial virus genetic diversity. Ther Adv Infect Dis. 2022;9:20499361221128091.
- [CrossRef] [PubMed] [Google Scholar]
- Severe respiratory syncytial virus infection in children: burden, management, and emerging therapies. Lancet. 2024;404:1143-56.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory syncytial virus infection and novel interventions. Nat Rev Microbiol. 2023;21:734-49.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in the application of molecular diagnostic techniques for the detection of infectious disease pathogens. Mol Med Rep. 2023;27:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory syncytial virus: A review of current basic and clinical knowledge. Qatar Med J. 2024;2024:56.
- [CrossRef] [PubMed] [Google Scholar]
- RSV genomic diversity and the development of a globally effective RSV intervention. Vaccine. 2021;39:2811-20.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances in diagnosis, prevention, and treatment of human respiratory syncytial virus. Adv Virol. 2013;2013:595768.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory syncytial virus entry mechanism in host cells: A general overview. Mol Microbiol. 2023;120:341-50.
- [CrossRef] [PubMed] [Google Scholar]
- Dimerization of matrix protein is required for budding of respiratory syncytial virus. J Virol. 2015;89:4624-35.
- [CrossRef] [PubMed] [Google Scholar]
- Revisiting respiratory syncytial virus's interaction with host immunity, towards novel therapeutics. Cell Mol Life Sci. 2020;77:5045-58.
- [CrossRef] [PubMed] [Google Scholar]
- A novel host factor for human respiratory syncytial virus. Commun Integr Biol. 2017;10:e1006062.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory syncytial virus: Biology, genetic diversity, and perspective control preparations. Mol Genet Microbiol Virol. 2024;39:14-30.
- [CrossRef] [Google Scholar]
- Molecular epidemiology and characteristics of respiratory syncytial virus among hospitalized children in Guangzhou, China. Virol J. 2023;20:272.
- [CrossRef] [PubMed] [Google Scholar]
- Biochemistry of the respiratory syncytial virus L protein embedding RNA polymerase and capping activities. Viruses. 2023;15:341.
- [CrossRef] [PubMed] [Google Scholar]
- Evolution and molecular epidemiology of respiratory syncytial virus genotypes: Global implications. J Infect Dis. 2023;228:1875-84.
- [Google Scholar]
- Structure-based design of respiratory syncytial virus vaccines targeting the prefusion F protein. Nat Rev Immunol. 2024;24:45-58.
- [Google Scholar]
- Clinical significance of respiratory syncytial virus genetic variants: Association with disease severity. Pediatr Infect Dis J. 2024;43:154-60.
- [Google Scholar]
- Enhanced replication efficiency of RSV-B BA9 genotype in human airway epithelial cells: Implications for pathogenesis. Viruses. 2023;15:2345.
- [Google Scholar]
- Global respiratory syncytial virus surveillance project based on the influenza platform Geneva: WHO; 2023. Available from: https://www.who.int/publications/i/item/who-strategy-for-global-respiratory-syncytial-virus-surveillance-project-based-on-the-influenza-platform [Last accessed on 2024 Jan 14]
- [Google Scholar]
- Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390:946-58.
- [Google Scholar]
- Respiratory syncytial virus infection among children younger than 2 years admitted to a paediatric intensive care unit with extended severe acute respiratory infection in ten Gavi-eligible countries: The RSV GOLD-ICU Network study. Lancet Glob Health. 2024;12:e1611-9.
- [CrossRef] [PubMed] [Google Scholar]
- 2024-2025 Respiratory disease season outlook. Atlanta, GA: CDC; 2024. Available from: https://www.cdc.gov/flu/about/season/flu-season.htm [Last accessed on 2024 Jan 14]
- [Google Scholar]
- Respiratory syncytial virus seasonality: A global overview. J Infect Dis. 2018;217:1356-64.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory syncytial virus infection trend is associated with meteorological factors. Sci Rep. 2020;10:10931.
- [CrossRef] [PubMed] [Google Scholar]
- Global distribution of respiratory syncytial virus A and B infections: A systematic review. Pathog Glob Health. 2022;116:398-409.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and transmission of respiratory syncytial virus in urban and rural South Africa, 2017-2018. Nat Commun. 2024;15:116.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of respiratory syncytial virus infection in Indian children: A multicenter surveillance study. Indian J Pediatr. 2023;90:782-9.
- [Google Scholar]
- Epidemiological patterns of RSV infections across six Indian states: Analysis of hospitalization rates and risk factors. J Glob Health. 2024;14:4008.
- [Google Scholar]
- Molecular surveillance of respiratory syncytial virus in India: Emergence and spread of novel genotypes. J Med Virol. 2024;96:e27788.
- [Google Scholar]
- Modelling respiratory syncytial virus age-specific risk of hospitalisation in term and preterm infants. BMC Infect Dis. 2024;24:510.
- [CrossRef] [PubMed] [Google Scholar]
- Socioeconomic impact of RSV hospitalization. Infect Dis Ther. 2021;10(Suppl 1):35-45.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory syncytial virus genotypes, host immune profiles, and disease severity in young children hospitalized with bronchiolitis. J Infect Dis. 2018;217:24-34.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory syncytial virus: Infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30:277-319.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular characterization of human respiratory syncytial virus in Seoul, South Korea, during 10 consecutive years, 2010-2019. PLoS One. 2023;18:e0283873.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and diagnostic accuracy of respiratory pathogens in pediatric populations: Insights from global studies. Cureus. 2024;16:e68652.
- [CrossRef] [Google Scholar]
- Molecular testing for respiratory viruses In: Diagnostic molecular pathology. United States: Academic Press; 2024. p. :117-32.
- [CrossRef] [Google Scholar]
- Identification of distinct genotypes in circulating RSV A strains based on variants in the virus replication-associated genes. J Virol. 2024;98:e0099024.
- [CrossRef] [PubMed] [Google Scholar]
- Differences between RSV A and RSV B subgroups and implications for pharmaceutical preventive measures. Infect Dis Ther. 2024;13:1725-42.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of anti-RSV vaccination in preventing respiratory syncytial virus disease and severe illness in older adults: A systematic review of randomized controlled trials. Eur Geriatr Med. 2024;15:1215-29.
- [CrossRef] [Google Scholar]
- Safety and efficacy of GSK’s RSVPreF3 vaccine in older adults: Phase 3 trial results. N Engl J Med. 2024;390:148-59.
- [Google Scholar]
- Maternal RSV vaccination with Pfizer’s bivalent prefusion F vaccine: Protection of young infants. Lancet. 2024;403:234-46.
- [Google Scholar]
- First mRNA-based respiratory syncytial virus vaccine: Safety and efficacy in adults. Vaccine. 2024;42:738-47.
- [Google Scholar]
- Development of live-attenuated respiratory syncytial virus vaccines for pediatric populations. J Pediatric Infect Dis Soc. 2024;13:31-42.
- [Google Scholar]
- Novel adenovirus vector-based respiratory syncytial virus vaccines: Phase 1/2 trial results. Vaccine. 2024;42:456-65.
- [Google Scholar]
- Combined influenza-RSV vaccination approaches: Clinical development and future prospects. Expert Rev Vaccines. 2024;23:89-102.
- [Google Scholar]
- Single-dose nirsevimab for prevention of RSV in infants: Real-world effectiveness data. N Engl J Med. 2024;390:367-78.
- [Google Scholar]
- Next-generation monoclonal antibodies for respiratory syncytial virus: Development and accessibility considerations. Nat Rev Drug Discov. 2024;23:55-70.
- [Google Scholar]
- Advancing RSV surveillance and disease burden as part of an expanded global influenza surveillance and response system Geneva: WHO; 2024. Available from: https://www.who.int/news-room/events/detail/2024/04/22/default-calendar/advancing-rsv-surveillance-and-disease-burden-as-part-of-an-expanded-global-influenza-surveillanceand-response-system [Last accessed on 2024 Jan 14]
- [Google Scholar]
- FDA approves RSV vaccine in pregnancy to protect infants The Washington Post; 2023.
- [Google Scholar]








