Translate this page into:
Histopathological spectrum of otorhinolaryngology tissue biopsies

*Corresponding author: Rimi Ibrahim Maryam, Medical Laboratory Science, Kaduna State University Kaduna, Kaduna, Nigeria. maryambdw@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Maryam RI, Salisu U, Suleiman H, Kabir M, Shuaib A, Abu N. Histopathological spectrum of otorhinolaryngology tissue biopsies. Adesh Univ J Med Sci Res. 2024;6:86-95. doi: 10.25259/AUJMSR_4_2024
Abstract
Objectives:
Otorhinolaryngology also known as ear, nose, and throat (ENT) is a surgical subspecialty within medicine that deals with the surgical and medical care of disorders of the ENT as well as the head and neck. Doctors obtain biopsy tissues from a patient in the theater or clinic through an open or endoscopic approach for histopathological analysis by a team of scientists and pathologists through effective grossing, processing, and microscopy.
Material and Methods:
This research aimed to analyze retrospectively histopathological diagnoses in the National Ear Care Centre, Kaduna. The objectives were to establish the demographic data of the patients and to determine the cases of ENT tissues for 10 years (from 2013 to 2022).
Results:
A total of 2394 biopsies were obtained at the National Ear Care Centre over the study period. 1244 (52%) were males, whereas 1150 (48%) were females, all within the age range of <1–70 years. Histopathology showed that 1543 (64.5%) of the cases were inflammatory/reactive while 21 (1%) were specific infections including fungal and viral. Of the neoplastic cases, 602 (25%) were benign, while 228 (9.5%) were malignant. Benign lesions involved cases such as lipoma, goiter, cysts, follicular adenoma, and squamous papilloma, which is the most occurring among others, while the cancerous lesions included cases such as malignant endothelioma, plasmacytoma, high-grade glioma, Bowen’s disease, fibrosarcoma, lymphoma, neuroblastoma, mature cystic teratoma, and carcinoma which is the most occurring.
Conclusion:
Histopathological diagnosis revealed promising results in aiding diagnosis, treatment, and prognosis of the varieties of lesions affecting patients referred to the hospital.
Keywords
Benign
Inflammatory
Malignancy
Otorhinolaryngology
Tumor
INTRODUCTION
Otology and neurotology focus on diseases affecting the outer, middle, and inner ear, as well as adjacent structures such as the facial nerve and lateral skull base. Conditions of the outer ear include otitis externa and ear canal inflammation. Middle ear disorders include otitis media, perforated eardrum (caused by infections, trauma, or loud noise), and mastoiditis. Inner ear diseases include benign paroxysmal positional vertigo, labyrinthitis, vestibular neuronitis, Ménière’s disease, perilymphatic fistula, acoustic neuroma, and vestibular schwannoma. Facial nerve conditions, such as Bell’s palsy also falls under this specialty.
Rhinology deals with nasal and sinus disorders, such as nasal septum deviation, nasal obstruction, sinusitis (acute or chronic), environmental allergies, rhinitis, pituitary tumors, empty nose syndrome, and severe or recurrent nosebleeds. Laryngology covers disorders like:
Dysphonia/hoarseness
Laryngitis
Reinke’s edema
Vocal cord nodules and polyps[1]
Spasmodic dysphonia
Tracheostomy
Laryngeal cancer
Vocology – the study and practice of voice habilitation.[2]
One of the most frequently performed ear, nose, and throat (ENT)surgeries is tonsillectomy, with or without adenoidectomy. These procedures are generally done to treat chronic infections or obstructive conditions, though in some cases, they may be required for suspected malignancies.[3] When neoplasia is suspected, pathological examination of the removed tissue is necessary.[4] However, there is ongoing debate about whether routine histological analysis is needed for non-neoplastic tonsillectomy or adenoidectomy specimens.[5-7]
Otorhinolaryngology (ORL), also referred to as otolaryngology head-and-neck surgery or simply ENT, is a medical subspecialty focused on both surgical and non-surgical management of conditions affecting the head and neck.[8] Physicians specializing in this field are called otorhinolaryngologists, otolaryngologists, head-and-neck surgeons, or ENT doctors. Common symptoms of ENT disorders include hearing loss, tinnitus (ringing in the ear), aural fullness (ear pressure sensation), otalgia (ear pain), otorrhea (ear discharge), vertigo, and imbalance.[9]
ENT specialists diagnose and treat conditions affecting the ENT, skull base, head, and neck. Functional disorders in these areas can impact essential functions such as breathing, speaking, swallowing, hearing, and eating.[10] In addition, ENT surgery includes procedures for treating tumors, reconstructing the head and neck, and performing cosmetic surgery on the face and neck. Surgical intervention may be required for conditions such as head-and-neck cancers, including mucosal, oral, oropharyngeal, laryngeal, hypopharyngeal, sinonasal, nasopharyngeal, skin, thyroid, salivary gland, and sarcomas. Endocrine surgeries related to ENT include thyroid and parathyroid surgery, microvascular free flap reconstruction, and skull base surgery.
Microvascular reconstruction
Microvascular reconstructive surgery is a key procedure in ORL.[11] This technique involves transferring a composite tissue graft from another part of the body to the head or neck. It is commonly used to reconstruct areas affected by cancers of the larynx, pharynx, oral cavity, salivary glands, jaw, skull, sinuses, tongue, and skin. The transferred tissue, often taken from the arms, legs, or back, may consist of skin, muscle, fat, or bone.[2] The choice of tissue depends on the patient’s reconstructive needs.
This technique allows for the restoration of jaw structure, improved tongue movement, and throat reconstruction. For the transplanted tissue to survive, it must establish a new blood supply.[12] After the surgery, the blood vessels of the graft are connected to those in the neck.[13,14] Since these blood vessels are very small (1–3 mm in diameter), a microscope is used for precise connections, making this a form of microvascular surgery.[15]
Statement of the problem
To the best of our knowledge, no retrospective studies of patients’ tissue specimens referred for histopathological analysis have been previously in the center. Therefore, this study is going to highlight the type of tissue specimens received and their respective histological diagnosis, which may influence health policies, particularly in this locality if need be. The frequency of each type of diagnosis established must be determined, particularly for cancer cases, which are quickly increasing in the modern world. Global health is a trendy concept that appears frequently in the headlines of the 21st century but is rarely comprehended by ENT professionals and patients. Despite increasing public health concerns regarding non-communicable diseases, particularly ENT problems, these disorders continue to receive little attention on the global health stage. Non-communicable diseases account for 60% of global mortality in 2008 even though most of the ENT cases were of benign nature and a large number of patients are affected at a given time.[16]
Aim
This research aims to fill the gap of knowledge about types of histopathological diagnosis of ENT post-surgical cases in the National Ear Care Centre, Kaduna.
Objectives
To establish the demographic data of patients
To determine histopathological diagnoses of ENT tissues retrospectively (2013–2022)
To classify the diagnoses based on the World Health Organization’s classification.
MATERIAL AND METHODS
Study design and location
Description of the study area
This study was conducted in the National Ear Care Centre, Kaduna State. Kaduna State is located in Northwest of Nigeria; it has a total area of 46,053 km2 (17,781 sq mi) and an area rank of 4th of the 36 states of Nigeria. There are about 9,000,000 million people going by 2022 census leaving it the 3rd of the 36 states of Nigeria in rank and a density of 130 km2 (340/sq mi). Its coordinates are 10°201 N and 7°451 E; this indicates the connection to the major routes reaching most of the states of the Nation. Kaduna State, north-central Nigeria, is politically classified as belonging to the now “North – West” zone of the current six Geo-political zones. It is populated by about 59–63 different ethnic groups where Gwari, Hausa, and Fulani are the dominant ethnic groups. Its water supply is sourced through damping of rivers and digging of wells and boreholes. Kaduna State consists of 23 local government areas [Figure 1].
- Map of Nigeria showing the location of Kaduna State.
- Source: Image adopted from researchgate.net
Ethics statement
Ethical approval was obtained from the training, ethical, and research committee of the National Ear Care Centre, Kaduna. Approval Date: 01/2021
Sample population
The population included all patients’ records referred for the histopathological analysis conducted in National Ear Care Centre, Kaduna.
Sample collection and processing
Conducted according to the Departmental Standard Operating Procedures, patients’ biodata without their identity were obtained from the departmental register and photomicrographs of cases were snapped and edited using the newly installed optika microscope and its attached screen.
Data analysis
Descriptive statistics such as numbers, proportions, and medians were used to calculate and summarize the sociodemographic data. All diagnoses were ascertained by a Consultant Pathologist.
RESULTS
Table 1 represents the age distribution of patients with age range of 0–9 years having the highest number with 1056 (48%), 10–19 years had a total 279 (13%), 20–29 years with 226 (10%), 30–39 years had 218 (10%), 40–49 years with 152 (7%), followed by 50–59 years with a total number of 127 (6%), then the age range of 60–69 with 83 (4%) and lastly above 70 with the least of 48 (2%) in number. Unspecified age distribution was 206 in total and the total number of patients that had undergone surgery was 2394.
| Agerange/years | 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | >70 | Total | Percentage distribution |
|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 100 | 27 | 30 | 12 | 11 | 10 | 3 | 3 | 196 | 9 |
| 2014 | 89 | 20 | 24 | 20 | 9 | 5 | 4 | 1 | 172 | 8 |
| 2015 | 109 | 28 | 21 | 28 | 10 | 4 | 6 | 3 | 209 | 10 |
| 2016 | 115 | 25 | 15 | 15 | 11 | 7 | 3 | 4 | 195 | 9 |
| 2017 | 115 | 24 | 17 | 14 | 8 | 7 | 5 | 4 | 194 | 9 |
| 2018 | 105 | 17 | 13 | 17 | 12 | 12 | 10 | 1 | 187 | 9 |
| 2019 | 79 | 19 | 17 | 14 | 10 | 6 | 6 | 3 | 154 | 7 |
| 2020 | 60 | 16 | 14 | 11 | 12 | 12 | 8 | 6 | 139 | 6 |
| 2021 | 114 | 50 | 31 | 44 | 33 | 22 | 17 | 8 | 319 | 15 |
| 2022 | 170 | 53 | 44 | 43 | 36 | 42 | 21 | 15 | 424 | 19 |
| Grand total (%) | 1056 (48) | 279 (13) | 226 (10) | 218 (10) | 151 (7) | 127 (6) | 83 (4) | 48 (2) | 2394 (Unspecified 205) | 100 |
Table 1 again indicates the yearly distribution of patients which included first 2013 with a total of 196 (9%), followed by 2014 with a total of 172 (8%), then 2015 with 209 (10%), 2016 with 195 (9%), then 2017 with 194 (9%), where we then had 2018 with a sum of 187 (9%), followed by 154 (7%) obtained in 2019, then 139 (6%) in 2020, 319 (15%) in 2021, and then finally 2022 with the highest figure of 424 (19%).
Table 2 indicates the number of males and females in the study subjects where there was a total of 1136 (52%) males referred for ENT surgery and on the other hand, 1041 (47%) females during the course of the study out of which two hundred and seventeen (8%) were not specified.
| Years | Male | Percentage | Female | Percentage |
|---|---|---|---|---|
| 2013 | 104 | 9 | 83 | 8 |
| 2014 | 95 | 8 | 77 | 7 |
| 2015 | 111 | 10 | 98 | 9 |
| 2016 | 96 | 8 | 99 | 9 |
| 2017 | 98 | 9 | 96 | 9 |
| 2018 | 106 | 9 | 81 | 8 |
| 2019 | 90 | 8 | 64 | 6 |
| 2020 | 66 | 6 | 73 | 7 |
| 2021 | 162 | 14 | 157 | 15 |
| 2022 | 208 | 18 | 213 | 20 |
| Unspecified | 217 | |||
| Total | 1136 | 1041 | 2177 | |
| Grand total | 2394 |
Table 3 represents the occurrences of inflammatory lesions with obstructive sialadenitis occurring 3 (0.2%) times, allergic rhinitis occurring 1 (0.1%) time, followed by reactive hyperplasia with the most frequent of 1443 (94%) times that included lymphadenitis, chronic suppurative, chronic sialadenitis, and otitis media, and the second most occurring was chronic non-specific inflammation with a total number of 62 (4%), then inflamed granulation tissue with a number of 30 (2%) that again included the inflamed type, pyogenic granuloma, chronic granulomatous inflammation, and keratin. Finally, preauricular sinuses occur 4 (0.3%) times. The total sum of all inflammatory lesions summed up to 1543 (64%) out of the 2394 cases recorded.
| Serial number | Diagnosis | Number | Percentage distribution |
|---|---|---|---|
| 1 | Obstructive sialadenitis | 3 | 0.2 |
| 2 | Allergic rhinitis | 1 | 0.1 |
| 3 | Reactive hyperplasia | 1443 | 94 |
| 4 | Chronic nonspecific inflammation | 62 | 4 |
| 5 | Inflamed granulation tissue | 30 | 2 |
| 6 | Preauricular sinuses | 4 | 0.3 |
| Grand total | 1543 | 100 |
Table 4 shows the total number and distribution of infectious lesions with rhinomucormycosis and verrucae (vulgaris and Palmacus) occurring 6 (38%) times each followed by molluscum contagiosum, tuberculosis, mucormycosis and actinomycosis occurring only 1 (6%) time each which gives a total number of 16 (1%) of the total cases.
| Serial number | Diagnosis | Number of cases | Percentage distribution |
|---|---|---|---|
| 1 | Rhinomucormycosis | 6 | 38 |
| 2 | Verruca (vulgaris and palmacus) | 6 | 38 |
| 3 | Molluscum contagiosum | 1 | 6 |
| 4 | Tuberculosis | 1 | 6 |
| 5 | Mucormycosis | 1 | 6 |
| 6 | Actinomycosis | 1 | 6 |
| Grand total | 16 | 100 |
Table 5 indicates the benign cases obtained during the course of this study with the most variety, 99 (16.6%) of which was squamous papilloma that included several types such as inflammatory, Schneiderian, inverted, squamous and sinonasal, 47 (7.9%) were follicular adenoma, 56 (9.4%) were cysts that were of different types that included keratinous, dentigerous, thyroglossal, bronchial, epidermal, retention, vocal fold, marictinus and then the frontoethmoidal type, 89 (14.9%) were goiter which were either colloid or nodular, 12 (2%) were lipoma which included the fibro and the cystic type, Laryngeal nodule which also included laryngocele occurred only 3 (0.5%) times, keloid occurred 17 (4%) times, hemangioma occurred twenty five 24 (2.8%) times with different types that included the ulcerated capillary, hematoma lobular, cystic lymphangioma and glomangioma, ameloblastoma and accessory tragus both occurred twice 2 (0.3%), while seborrheic keratosis, glomus tumor, ear hydrocystoma, cellular schwannoma, tumor of precancer, lump of post auricular, Sino nasal tract meningioma, olfactory pseudo membrane and adenomatoid odontogenic tumor all occurred once, 1 (0.16%) in 10 years, 7 (1.2%), severe dysplasia and fibroma (which included the chondromyxoid, ossifying, neuro and fibromatosis) both occurred 7 (1.2%) times while cholesteatoma occurred 14 (4%) times, esophageal tumor occurred 4 (0.7%) times and lastly polyps occurred 205 (34%) times which included the fibroepithelial, inverted, allergic nasal, inflammatory, laryngeal, inflamed nasal, vocal cord antrochoanal and angiomatosis. The total number of benign lesions out of the total is five hundred and ninety seven (25%) of the total diagnoses.
| Serial number | Diagnosis | Number of Cases | Percentage Distribution |
|---|---|---|---|
| 1 | Squamous papilloma | 99 | 16.6 |
| 2 | Follicular adenoma | 47 | 7.9 |
| 3 | Cysts | 56 | 9.4 |
| 4 | Goiter | 89 | 14.9 |
| 5 | Lipoma | 12 | 2 |
| 6 | Laryngeal nodule | 3 | 0.5 |
| 7 | Keloid | 17 | 4 |
| 8 | Hemangioma | 24 | 2.8 |
| 9 | Ameloblastoma | 2 | 0.3 |
| 10 | Seborrheic keratosis | 1 | 0.16 |
| 11 | Severe dysplasia | 7 | 1.2 |
| 12 | Fibroma | 7 | 1.2 |
| 13 | Cholesteatoma | 14 | 2.3 |
| 14 | Glomus tumor | 1 | 0.16 |
| 15 | Esophageal tumor | 4 | 0.7 |
| 16 | Ear hidrocystoma | 1 | 0.16 |
| 17 | Accessory tragus | 2 | 0.3 |
| 18 | Cellular schwannoma | 1 | 0.16 |
| 19 | Tumor of precancer | 1 | 0.16 |
| 20 | Lump of postauricular | 1 | 0.16 |
| 21 | Sino nasal tract meningioma | 1 | 0.16 |
| 22 | Olfactory pseudo membrane | 1 | 0.16 |
| 23 | Adenomatoid odontogenic tumor | 1 | 0.16 |
| 24 | Polyps | 205 | 34 |
| Grand total | 597 | 100 |
Table 6 shows the malignant lesions from patients during the course of this study with carcinoma occurring 203 (89%) times with several types, which included the poorly differentiated squamous cell carcinoma, nasopharyngeal, adenoid cystic, papillary, squamous, acini cell, lymphoepithelial, adenocarcinoma, thyroid, anaplastic, metastatic hepatocellular, well-differentiated squamous cell carcinoma and metastatic, followed by rhabdomyosarcoma occurring 11 (5%) times and the alveolar type was recorded, matured cystic teratoma and Bowen’s disease occurring 2 (1%) times each, neuroblastoma and lymphoma (the non-Hodgkin’s type) both occurring 3 (1%) times each, and finally fibrosarcoma, high-grade glioma, malignant endothelioma, and plasmacytoma all occurring only 1 (0.4%). These all form a total of 228 (10%) of the 2394 cases obtained in 10 years.
| Serial number | Diagnosis | Number of cases | Percentage distribution |
|---|---|---|---|
| 1 | Carcinoma | 203 | 89 |
| 2 | Rhabdomyosarcoma | 11 | 5 |
| 3 | Mature cystic teratoma | 2 | 1 |
| 4 | Neuroblastoma | 3 | 1 |
| 5 | Lymphoma | 3 | 1 |
| 6 | Fibrosarcoma | 1 | 0.4 |
| 7 | Bowen’s disease | 2 | 1 |
| 8 | Highgrade glioma | 1 | 0.4 |
| 9 | Plasmacytoma | 1 | 0.4 |
| 10 | Malignant endothelioma | 1 | 0.4 |
| Grand total | 228 | 100 |
The last table that is Table 7 shows specified and unspecified diseases of cells with Langerhans histiocytosis occurring only 1 (10%), unspecified occurring 7 (70%) times, and lastly olfactory pseudomembrane formation making a total number of 2 (20%). These all together form a total of 10 out of the 2394 cases identified.
| Serial number | Diagnosis | Number of cases | Percentage distribution |
|---|---|---|---|
| 1 | Langerhans cell histiocytosis | 1 | 10 |
| 2 | Olfactory pseudomembrane | 2 | 20 |
| 3 | Unspecified | 7 | 70 |
| Grand total | 10 | 100 |
Mean median and standard deviation of the demographic studies of patients include as follows:
Detailed statistical summary, thus calculated as:
Mean (Average) Total Cases Per Year = (196 + 172 + 209 + 195 + 194 + 187 + 154 + 139 + 319 + 424)/10 = 208.9 cases
-
Median Total Cases Per Year = The middle value when the totals are ordered:
Ordered: 139, 154, 172, 187, 194, 195, 196, 209, 319, 424
Median = (194 + 195)/2 = 194.5 cases
Standard Deviation: This measures the spread of the values around the mean.
-
Calculating the standard deviation for these values:
Standard deviation (σ) = √[(Σ (xi - μ)2)/N]
For simplicity, this calculation will be based on the formula
Standard Deviation ≈ 79.55 cases.
Per Age Range (Across All Years):
The age range values were calculated across all years.
-
For the 0–9 Age Range as an example:
Total cases for 0–9: 100 + 89 + 109 + 115 + 115 + 105 + 79 + 60 + 114 + 170 = 1,097 cases
Mean = 1,097/10 = 109.7 cases
Median: Since it’s a simple list: 100, 89, 109, 115, 115, 105, 79, 60, 114, 170
Median = (109 + 115)/2 = 112 cases
Standard Deviation ≈ 32.4 cases.
-
Example of Age Range 0–9:
This age group had 100 cases in 2013, and by 2022, it had 170 cases, which is an increase. However, we should also notice its percentage as part of the total fluctuates, dropping from 9% in 2013 to around 4% in 2022.
There is a decline in percentage from 2013 to 2020 but a noticeable increase in 2021 and 2022. This could suggest a trend shift in the population of cases from younger to older age groups over time.
Total cases: Fluctuated but showed a significant rise in 2021 and 2022.
Age group shifts: Younger age groups (like 0–9) had a higher percentage in earlier years, but older groups (particularly >70) show an uptick over time.
Data spread: The standard deviation suggests variability in cases each year, with higher variance in the early years compared to the later years. Some of the photomicrographs were obtained [Figures 2-10].
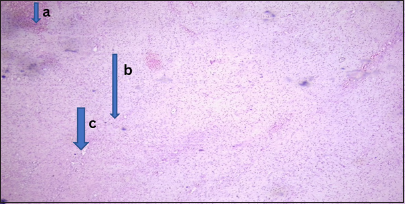
- Benign lesion (myxoid fibroma). Photomicrograph of section from the nasal mass showing (a) pseudostratified columnar epithelium. (b) The stroma showed sheets of spindle-shaped cells, round-to-oval cells having bland nuclei with scanty cytoplasm and (c) few medium sized vascular channels. (Magnification: ×10 Hematoxylin and Eosin stain)
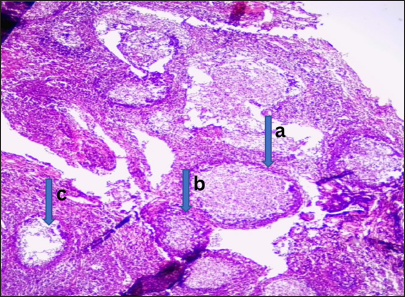
- Malignant lesion (well-differentiated nasopharyngeal carcinoma). Photomicrograph: Section obtained from the nasal mass shows (a) a tumor disposed as irregular nests and anastomosing trabeculae. (b) The comprising cells have hyperchromatic to vesicular nuclei, prominent nucleoli, and indistinct cytoplasm. (c) The stroma is desmoplastic with extensive lymphocytic infiltrates. (Magnification: ×100 Hematoxylin and Eosin stain)
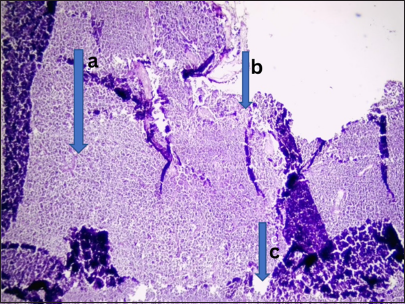
- Malignant lesion (non-Hodgkin’s lymphoma). Photomicrograph of section from lymph node tissue shows, (a) effaced architecture by infiltrating sheets of small lymphoid cells predominantly enterocytes having hyperchromatic vesicular nuclei with scanty cytoplasm. (b) These are disposed within lymphoid stroma. Features are those of non-Hodgkins lymphoma. (c) Small lymphocytic lymphoma highly favored under Mantoux cell lymphoma. (Magnification: ×40 Hematoxylin and Eosin stain)
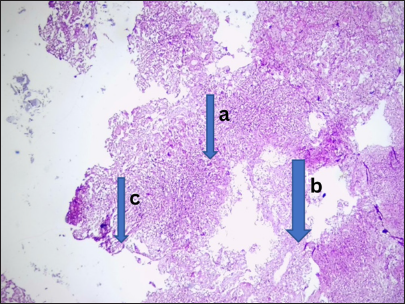
- Infectious lesion (rhinomucormycosis). Photomicrograph of section from the fronto-ethnoidal tissue shows (a) several multinucleated giant cells, (b) several fungal hyphae and histiocytes. (c) These are disposed within fibrocollagenous stroma. Features were those of rhinomucormycosis. (Magnification: ×100 Hematoxylin and Eosin stain)
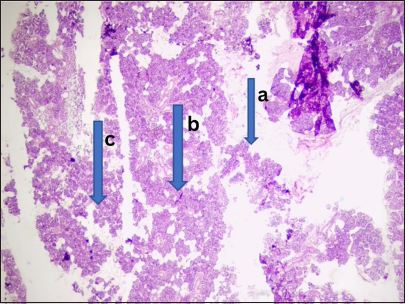
- Keratinizing nasopharyngeal carcinoma. Photomicrograph: The nasopharyngeal tissue showed (a) a gamma disposed in a small nest and comprising of cells which are medically presumed to be large around nucleic, (b) prominent nucleic and moderate cytoplasm, it again showed individuals cells dyskeratosis. (c) These are disposed within a background of extensive inflammation and necrosis. (Magnification: ×100 Hematoxylin and Eosin stain)
- Metastatic papillary thyroid carcinoma. Photomicrograph of the sections from the cervical lymph node shows (a) infiltrating nests and sheets of discohesive clusters of atypical follicular epithelial cells. The cells exhibit marked pleomorphism having hyperchromatic nuclei and orphan Annie eye appearance. (b) Brisk mitosis was noted and few unremarkable variable sized colloid follicles were seen. (c) The accompanying lymph node demonstrates similar atypical follicular cells. (Magnification: × 40 Hematoxylin and Eosin stain)
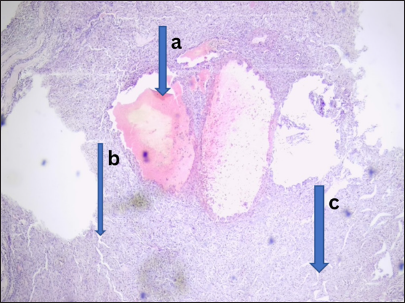
- Chronic granulomatous inflammation. Photomicrograph shows section of left cervical (a) swelling with several (b) granulomas composed of epithelial type giants’ cells; these are disposed within (c) caseous necrotic background. (Magnification: ×100 Hematoxylin and Eosin stain)
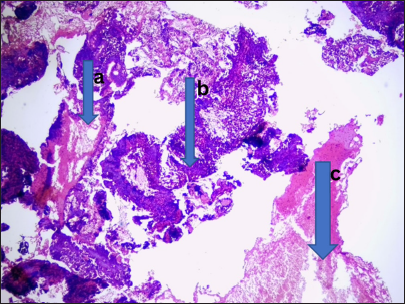
- Plasmacytoma. Photomicrograph from the cervical lymph nodes shows, (a) dense monotonous infiltrates of plasma cells ranging from mature binucleated to anaplastic with effaced nodal architecture. (b) These were traversed by collagenous trabeculae. (c) Numerous eosinophilic globules were seen, suggestive of Russell bodies. (Magnification: ×100 Hematoxylin and Eosin stain)
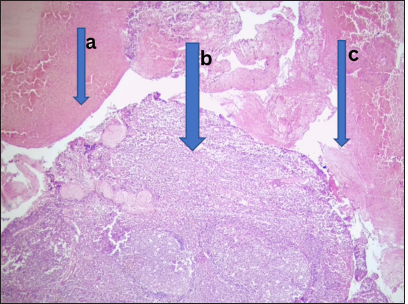
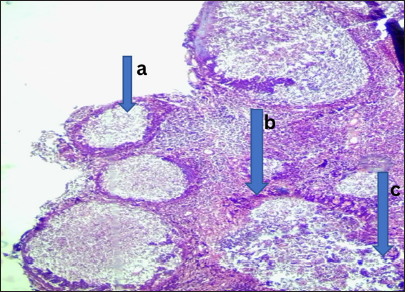
- Received lymphoid hyperplasia. Photomicrograph of section from the adenoids shows (a) hyperplastic lymphoid follicles with (b) prominent germinal center in addition to (c) extensive areas of hemorrhage. (Magnification: ×100 Hematoxylin and Eosin stain)
DISCUSSION
Similar to the gender distribution observed in this study, where a total of 2394 patients underwent surgery – 1136 (52%) males and 1041 (47%) females – previous literature reported that 1707 individuals underwent otorhinolaryngological procedures, comprising 918 males and 789 females. Chronic or recurrent infections, along with obstructive issues, were primary indications for these surgeries. In line with Table 3, which highlights inflammatory lesions, 435 patients (234 females and 201 males) underwent bilateral tonsillectomy, while adenoidectomy was performed in 502 patients (226 females and 276 males). These procedures were conducted at the Department of ORL, Inonu University Medical Faculty, Malatya, Turkey.[17]
Likewise, Table 1 reveals that the 0–9 age group had the highest number of surgeries. However, a 2005 study by Hegde et al. found that patients’ ages ranged from 2 to 68 years, with the third decade of life (42%) being the most affected, followed by the second (16%) and fourth (16%) decades.[17,18] Similar findings have been reported previously.[19]
In this study, the affected areas primarily included the ear, nose, head, and throat, with diagnoses indicating that vocal polyps were unilateral, totaling 205 recorded cases. Meanwhile, vocal nodules and Reinke’s edema were bilateral. False cord (vestibular fold) lesions were documented in four cases (8%).[20] In contrast, a 2009 study by Singhal et al. found that vocal cords were the most frequently involved site in 44 cases (88%), with other laryngeal regions less commonly affected. The true vocal cords were involved in 86% of cases, with right cord involvement (26%) slightly more common than left (20%), while 20 cases (40%) involved both cords. Epiglottis involvement was noted in two cases (4%), with one also affecting the right aryepiglottic fold. Another case involved the anterior commissure and part of the left vocal cord.[20] Regarding the exact site of vocal cord polyps and nodules, polyps were primarily located at the anterior third or at the junction of the anterior and middle thirds of the true vocal cords, whereas vocal nodules exclusively occurred at this junction. Reinke’s edema affected the entire length of the true vocal cords.[19]
Laryngeal nodules were observed in three cases, as indicated in Table 5. Meanwhile, microlaryngoscopic examination under anesthesia in another study involving 41 patients found seven cases of vocal nodules, which may be attributed to effective conservative treatment with vocal rest and speech therapy, as noted in a 2015 study by Sharma et al.[21] Rhinomucormycosis was identified in six cases (37.5%) in this study, comparable to one case in a 2005 study by Sharma et al that involved the tracheobronchial tree and was unresponsive to treatment.[21] Table 5 also shows diagnostic frequencies that differ slightly from a 2000 study by Padmakumary and Varghese, which reported 22 cases (44%) of polypoid lesions, 16 cases (32%) of vocal nodules, one case (2%) of a true vocal cord cyst, and three cases (6%) of Reinke’s edema, four cyst cases, and one case each of post-traumatic granuloma, laryngopyocele, papilloma, hemangioma, and laryngeal sporidiosis. Notably, a case initially diagnosed as a supraglottic cyst through indirect laryngoscopy was later confirmed as a laryngopyocele through direct microlaryngoscopy.[22]
Different from the findings in this study, Tables 1 and 6 indicate that an institute in Southern India documented 67,909 surgical biopsies in 2006, as reported by Saraswathi et al. Among these, 1,151 biopsies were from the oral region, with 365 (31.7%) being benign, 344 (29.8%) classified as potentially malignant, and 442 (38.4%) malignant. The prevalence rate was 15.58/1,000 biopsies, with 76.9% of cases occurring in males and 23% in females.[23] However, in this study, approximately 10% of cases were malignant, while about 90% were benign. Similarly, a study conducted over 18 years in Allahabad, India, analyzed 1151 oral biopsies, identifying 344 premalignant cases, with 73.2% occurring in males and 26.7% in females. Malignant cases accounted for 38.4%, diagnosed primarily as oral squamous cell carcinoma (OSCC). Among the benign cases, 221 were males and 144 were females.[18] In line with Table 1, no significant gender difference was observed in this study.
Padmakumary and Varghese reported that OSCC comprised 14% of all cancers at the Regional Cancer Center in Kerala, India, accounting for 17% of male cancers and 10.5% of female cancers.[22] The male-to-female ratio in this study was 2.4:1, comparable to the 2.3:1 ratio reported by Iype et al in 2001, while the present study found a ratio of approximately 1:1.[24] Consistent with previous observations, most oral malignancies were seen in the 50–59 age group, a trend also reported by Gangane et al.[25] However, Saraswathi et al found that most cases were in the 40–61 age range.[26] Among premalignant cases reported by Gangane et al, 252 were males and 92 were females. Among the 252 males, 41 had dysplasia, 67 had leukoplakia, and 144 had oral submucous fibrosis (OSMF), while among the 92 females, 17 had dysplasia, 23 had leukoplakia, and 52 had OSMF.[25]
In the malignant group of 442 patients, 188 males had well-differentiated carcinoma, while 141 had moderately differentiated carcinoma. Among females, 53 had well-differentiated carcinoma, and 42 had moderately differentiated squamous cell carcinoma. This totaled 203 carcinoma cases, comprising about 89% of all malignancies in this study.[26] According to age distribution, most benign and premalignant biopsies occurred in the 20–29 age group, with a mean age of 25 years, while malignant cases were primarily found in the 50–59 age group, with a mean age of 55 years.[27] In terms of site involvement, biopsies from the oral cavity showed that in the benign and premalignant group, the buccal mucosa was the most frequently affected area, seen in 260 (47.7%) patients, followed by the tongue in 26 (27.6%) cases.[28] However, for malignant cases, the tongue was the most affected site in 167 patients (37.8%), followed by the buccal mucosa in 149 cases (33.7%).[29-34]
Data interpretation from a single institution has limitations, as it reflects only the specific patient population of the hospital rather than the broader community. However, as the only ENT hospital in the northern region of the country, it provides a representative sample. This study revealed that surgeries peaked in 2022, followed by 2021, likely due to increased awareness and population growth. These findings have not been previously reported, aside from isolated studies on specific lesions by certain clinicians. Analyzing data trends from 2013 to 2022 suggests a gradual increase in various diagnoses, though more research is needed to understand the evolving patterns. The annual biopsy reports have distinct characteristics requiring further investigation, as this study provides only a general overview of surgical patterns in the center. The distribution of cases by clinical diagnosis – benign, malignant, inflammatory, and cell disease – varies in etiology, causes, and prognosis.
Recommendations
It is highly recommended that the use of screening and detection methods such as vital stains, visualization aids as well as brush biopsy should be reported to increase the number of cases diagnosed at an early stage, or even in the premalignant stage. It is again recommended to develop molecular markers in some certain conditions like epstein barr virus screening in elderly men to watch out for nasopharyngeal carcinoma that may improve the early diagnosis and help in predicting treatment response. New treatment modalities including tumor specific antibodies and gene therapy are emerging, giving more hope for patients with ENT cases. There is an urgent need for appropriate prevention and cessation strategies for all carcinogenetic predisposing lifestyle and substances.
CONCLUSION
Histopathological diagnosis revealed promising results in aiding patients’ diagnosis, prognosis, and treatment of the varieties of lesions affecting patients referred to the hospital during the 10-year period and beyond, and it also has successfully determined the spectrum of ORL tissue biopsies obtained in national ear care Centre, Kaduna.
Ethical approval
The research/study approved by the training, ethical, and research committee of the National Ear Care Centre, Kaduna, number NECC/ADM/214/IV/247, dated 01st January 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Pathologic analysis of routine tonsillectomy and adenoidectomy specimens. Otolaryngol Head Neck Surg. 2001;125:473-7.
- [CrossRef] [PubMed] [Google Scholar]
- Microscopic examination of routine tonsillectomy specimens: Is it necessary? Otolaryngol Head Neck Surg. 1998;119:361-3.
- [CrossRef] [PubMed] [Google Scholar]
- Routine pathological evaluation after tonsillectomy: Is it necessary? B-ENT. 2006;2:103-8.
- [Google Scholar]
- Incidence of unexpected pathology in routine adenoidectomy specimens. Int J Pediatr Otorhinolaryngol. 2006;70:95-8.
- [CrossRef] [PubMed] [Google Scholar]
- The histopathology of routine tonsillectomy specimens: Results of a study and review of literature. Ear Nose Throat J. 2000;79:880-2.
- [CrossRef] [PubMed] [Google Scholar]
- Pathologic evaluation of routine tonsillectomy and adenoidectomy specimens in the pediatric population: Is it really necessary? Int J Pediatr Otorhinolaryngol. 2005;69:1321-5.
- [CrossRef] [PubMed] [Google Scholar]
- Processing of adenoid and tonsil specimens in children: A national survey of standard practices and a five-year review of the experience at the children's hospital of Pittsburgh. Otolaryngol Head Neck Surg. 1996;115:94-7.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphatic spread of mesenchymal renal tumor to metastatic parathymic lymph nodes in rat. Histol Histopathol. 2009;24:1367-79.
- [Google Scholar]
- Otolaryngology Specialty Description. Philadelphia; PA: American Medical Association; Available from: https://freida.ama-assn.org/specialty/otolaryngology-head-and-neck-surg [Last accessed 2024 Jan 10]
- [Google Scholar]
- Rolul modificarilor corporale în identificarea victimelor în dezastre. Roma J Legal Med. 2008;16:128-33.
- [Google Scholar]
- Necessity of routine pathological examination of tonsils. Eye Ear Nose Throat Mon. 1972;51:229-30.
- [Google Scholar]
- Histological examination following adenoidectomy and tonsillectomy in children. Surprising results are very rare. HNO. 2006;54:16-9.
- [CrossRef] [PubMed] [Google Scholar]
- The adequacy of gross pathological examination of routine tonsils and adenoids in patients 21 years old and younger. Hum Pathol. 2003;34:1053-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pharyngitis and adenotonsillar disease In: Cummings CW, Fredrickson JM, Harker LA, Krause CJ, Schuller DE, eds. Pediatric otolaryngology head and neck surgery. Missouri: MosbyYear Book; 1998. p. :188-215.
- [Google Scholar]
- Histologic analysis of pediatric tonsil and adenoid specimens: Is it really necessary? Int J Pediatr Otorhinolaryngol. 2009;73:547-50.
- [CrossRef] [PubMed] [Google Scholar]
- Global progress in infectious disease control. Vaccine. 1998;16:1116-21.
- [CrossRef] [PubMed] [Google Scholar]
- Benign lesions of larynx-a clinical study. Ind J Otolaryngol Head Neck Surg. 2005;57:35-8.
- [CrossRef] [PubMed] [Google Scholar]
- Oral squamous cell carcinoma in 38 year old male: A case report. J Oral Med Oral Surg Oral Pathol Oral Radiol. 2020;6:92-7.
- [CrossRef] [Google Scholar]
- Benign tumors of larynx: A clinical study of 50 cases. Indian J Otolaryngol Head Neck Surg. 2009;61(Suppl 1):26-30.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical outcomes in laryngeal cancer involving the anterior commissure: A multicenter retrospective study. J Laryngol Otol. 2023;137:322-8.
- [Google Scholar]
- A clinical study of benign lesions of larynx. Int J Oral Health Med Res. 2015;2:22-8.
- [Google Scholar]
- Annual Report 1997 Hospital Cancer Registry Thiruvananthapuram, Kerala: Regional Cancer Centre; 2000. p. :3-7.
- [Google Scholar]
- Classification of Oral Squamous Carcinoma Histopathological images using Alex Net. 2023. International Conference on Intelligent Systems for Communication, IoT and Security (ICISCoIS) 2023:637-43.
- [CrossRef] [Google Scholar]
- Oral cancer among patients under the age of 35 years. J Postgrad Med. 2001;47:171-6.
- [Google Scholar]
- Reassessment of risk factors for oral cancer. Asian Pac J Cancer Prev. 2007;8:243-8.
- [Google Scholar]
- Prevalence of oral lesions in relation to habits: Cross-sectional study in South India. Indian J Dent Res. 2006;17:121-5.
- [CrossRef] [PubMed] [Google Scholar]
- Age specific incidence rate and pathological spectrum of oral cancer in Allahabad. Indian J Med Sci. 2003;57:400-4.
- [Google Scholar]
- Cancer of the oral cavity-trends in Karachi South (1995-2002) Asian Pac J Cancer Prev. 2005;6:22-6.
- [Google Scholar]
- Prevalence of oral pre-malignant and malignant lesions at a tertiary level hospital in Allahabad, India. Asian Pac J Cancer Prev. 2008;9:263-5.
- [Google Scholar]
- Evaluation of the utility and cost-effectiveness of obtaining histopathologic diagnosis on all routine tonsillectomy specimens. Laryngoscope. 2001;111:2166-9.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of unexpected malignancies in routine tonsillectomy specimens in children. Laryngoscope. 2004;114:1103-5.
- [CrossRef] [PubMed] [Google Scholar]
- Histological analysis of tonsillectomy and adenoidectomy specimens--January 2001 to May 2003. Braz J Otorhinolaryngol. 2005;71:18-22.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for malignancy in adult tonsils. Head Neck. 1998;20:399-40.
- [CrossRef] [Google Scholar]







