Translate this page into:
Fluoroquinolone antibiotics: An overview

*Corresponding author: Ramanjeet Kaur Brar, Department of Pharmaceutical Sciences, AIPBS, Bathinda, Punjab, India. ramanr64@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Brar RK, Jyoti U, Patil RK, Patil HC. Fluoroquinolone antibiotics: An overview. Adesh Univ J Med Sci Res 2020;2(1):26-30.
Abstract
Fluoroquinolones are the type of antibacterial agents more extensively used from the past few years and will be continued to be used even in the next decade. The fluoroquinolones show their action by inhibiting the DNA gyrase and topoisomerase enzymes. The main mechanism fluoroquinolones is mutations that alter the accumulation of fluoroquinolones in bacteria. The broad use of the fluoroquinolones is discussed. They are useful in the treatment of urinary tract infections, prostatitis, sexually transmitted diseases, gastrointestinal infections, osteomyelitis, and respiratory tract infections. Their structure–activity relationship is also discussed and that is helpful in ongoing new researches on quinolones that may enhance the antibacterial activity of quinolones.
Keywords
Antibacterial agent
Broad-spectrum antibiotic
Structure–activity relationship
DNA gyrase
Topoisomerase
Mutations
Future prospects/Directions
INTRODUCTION
Antimicrobial agents are chemical agents which are used to treat the infections by bacterial, viral, fungal, and microorganism infections. A variety of antimicrobial agents were used in the past decades to treat different types of infections. Beta-lactam antibiotic penicillins and sulfonamides were commonly used in clinical usage.[1] Later on, quinolones and fluoroquinolones were established as new class of synthetic antibiotics with broad-spectrum activity and potent bactericidal agents which are active against to important pathogens that cause the variety of infections including urinary tract infections (UTIs),[2] gastrointestinal infections (GITs), respiratory tract infections (RTIs), sexually transmitted diseases (STDs), and skin infections.[3,4]
The fluoroquinolones are a class of broad-spectrum antimicrobial agents, therefore, they are highly active against both aerobic Gram-positive and Gram-negative organisms. Gram-positive includes penicillinase and non-penicillinase-producing staphylococci, Streptococcus pneumoniae and Streptococcus viridans, Enterococcus faecalis, Listeria monocytogenes, and Nocardia species. Gram-negative includes Neisseria meningitides and Neisseria gonorrhoeae, Haemophilus influenzae, and most important Enterobacteriaceae species, Pseudomonas aeruginosa, and Vibrio species.[5]
In 1962, nalidixic acid, first clinically useful quinolone, was discovered by Lesher et al.[1] In 1980, an analog of nalidixic acid, enoxacin was derived with increased spectrum of activity against Gram-negative or Gram-positive bacteria.[6] In 1986, another fluoroquinolone drug that is ciprofloxacin was marketed,[1,7] due to its more pharmacokinetic property and potent activity against various pathogens.[7,8]
CLASSIFICATION OF FLUOROQUINOLONES
Fluoroquinolones are classified on the basis of their spectrum of activity [Table 1].[9-11]
| Based on origin | Name of drug | Dose regimen | Spectrum activity | Uses |
|---|---|---|---|---|
| First generation | Nalidixic acid Cinoxacin |
0.5–1 g TDS 500 mg BD oral[9] |
Gram-negative organisms except Pseudomonasspecies | Uncomplicated urinary tract infections[10] |
| Second generation | Norfloxacin Lomefloxacin Enoxacin Ofloxacin Ciprofloxacin |
400 mg BD oral[9] 400 mg OD oral[9] 200–400 mg BD oral[9] 250–750 mg BD oral[9] 250–750 mg BD oral[9] |
Gram-negative organisms and also Pseudomonasspecies, some Gram-positive organisms including Staphylococcus aureusexcept Streptococcus pneumoniaeand some atypical pathogens | Uncomplicated and complicated urinary tract infections, pyelonephritis, sexually transmitted diseases, prostatitis, skin and soft-tissue infections[11] |
| Third generation | Levofloxacin Sparfloxacin Gatifloxacin Moxifloxacin |
250–500 mg OD oral[9] 200 mg OD oral[9] 400 mg OD oral[9] 400 mg OD oral[9] |
Gram-negative organisms and Gram-positive and also penicillin-sensitive and penicillin-resistant Streptococcus pneumoniaeand expanded activity against atypical pathogens | Acute exacerbations of chronic bronchitis, community-acquired pneumonia[11] |
| Fourth generation | Trovafloxacin | 100–200 mg OD oral[9] | Gram-negative organisms and Gram-positive, penicillin-sensitive and penicillin-resistant Streptococcus pneumoniaeand expanded activity against atypical pathogens and broad anaerobic coverage | Same as for first-, second-, and third-generation agents (excludingcomplicated urinary tract infections and pyelonephritis) plus intra- abdominal infections, nosocomial pneumonia, pelvic infections |
STRUCTURE–ACTIVITY RELATIONSHIP (SAR)
Fluoroquinolones are synthetic fluorinated analogues of nalidixic acid, a 1,8-naphthyridine and possess a 4-quinolone nucleus.[11] The quinolone structure consists of a bicyclic system with a substituent at position N-1, a carboxyl group at position 3, a keto group at position 4, a fluorine atom at position 6, and a substituent (often nitrogen heterocycle moiety) at the C-7. Normally, in position 2, there are no substituents, various 1-methyl-2-alkenyl-4(1H).[12]
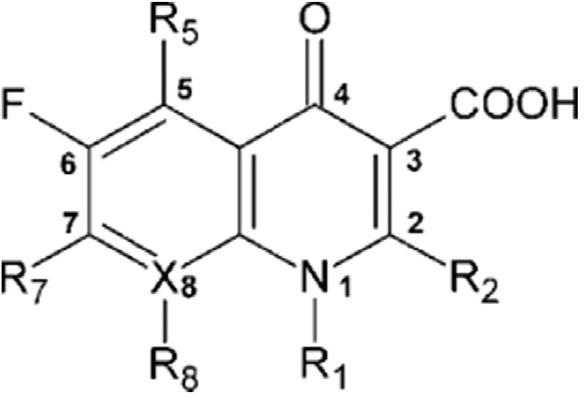
- 3-Fluro-2(1H)-quinolinone
The research on quinolones started in 1962 along with of nalidixic acid, the prototype 4-quinolone antibiotic.[13] The structural modification may enhance the antibacterial activity and pharmacokinetic properties. The nucleus of quinolone is consisting of nitrogen and 8-membered heterocyclic aromatic quinoline ring. In SAR of the early 4-quinolones, the 3-carboxyl group and 4-oxo groups were linked to antibacterial activity.[14]
MECHANISM OF ACTION
Fluoroquinolones show their action by inhibiting the replication and transcription of bacterial DNA that is responsible for proper functioning of the cell.[15,16] During DNA replication and transcription, double-stranded DNA goes to uncoil into a single-stranded structure by enzymes called DNA gyrase or DNA topoisomerase. DNA gyrase is an essential adenosine triphosphate-hydrolyzing topoisomerase II enzyme that prevents the detachment of gyrase from DNA. It consists of two A subunits (gyrA) and two B subunits (gyrB). DNA gyrase establishes negative super-helical twists in the bacterial DNA [Figure 1].[17] Quinolones and fluoroquinolones inhibit this enzyme by binding to the A subunit of the enzyme due to which the bacteria are unable to replicate or even synthesize proteins. There is DNA-binding groove between the A and B subunits so that binding of the fluoroquinolones to this groove may conformity change the DNA gyrase molecule. Then, DNA becomes another binding site itself, as a result fluoroquinolones bind with both DNA and DNA gyrase. In many bacteria, DNA gyrase acts as the primary site of fluoroquinolone action, including E. coli.[18] Fluoroquinolones have also been found to inhibit the in vitro activities of topoisomerase IV, having structure similar to DNA gyrase.[2] The 2nd target site for the fluoroquinolones is topoisomerase IV, this is made up from two ParC subunits (parC) and two ParE subunits (parE). The protein subunits coded for by parC (ParC) and parE (ParE) are homologous to the A and B subunits of DNA gyrase, respectively. Topoisomerase IV carries out decatenation and relaxation of DNA and assists with the segregation of replicating chromosomes or plasmids in bacteria.[19] The bactericidal activity of the fluoroquinolones is enhanced by inhibition of topoisomerase IV.
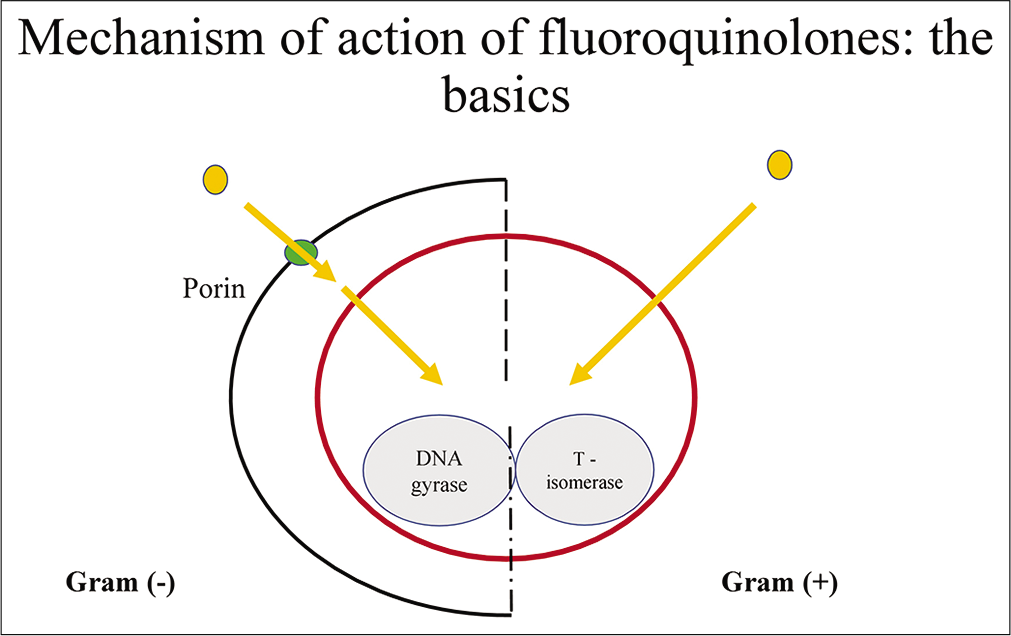
- Mechanism of action of fluoroquinolone.
MECHANISM OF RESISTANCE
Resistance to fluoroquinolones mostly occurs by two mechanisms that are mutations in the both target enzymes DNA gyrase in Gram-negative bacteria and topoisomerase IV in Gram-positive bacteria. The second way that reduced accumulation of the fluoroquinolones can occur is through an efflux system. Resistance is due to increased expression of chromosomal gene leading to increased efflux of the fluoroquinolones [Figure 2].[20]
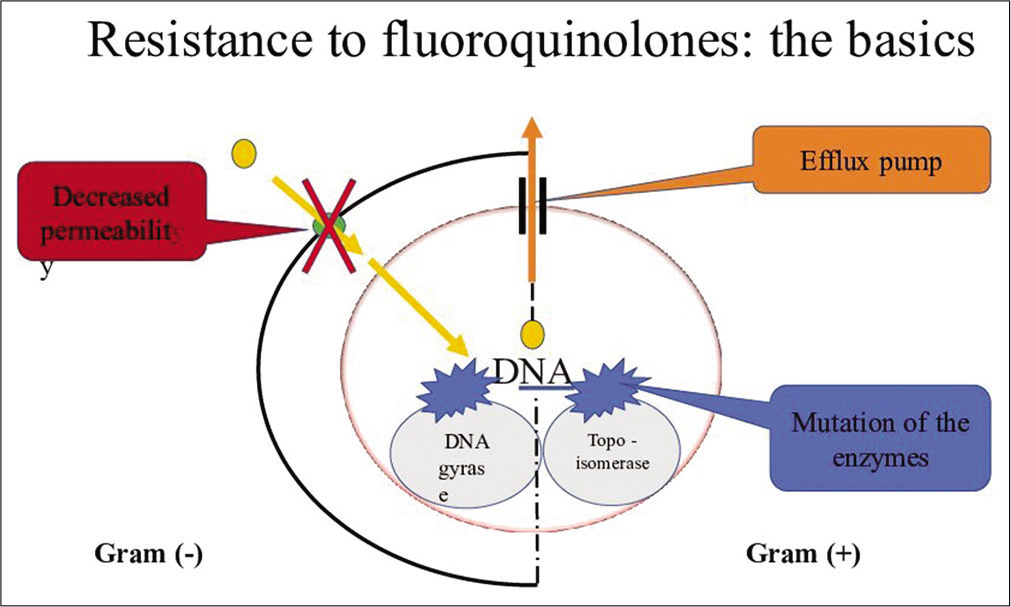
- Resistance to fluoroquinolone.
THERAPEUTIC USES
-
UTIs
Norfloxacin is used for UTIs and fluoroquinolones are more effective than trimethoprim-sulfamethoxazole for the treatment of UTIs.[21]
-
Prostatitis
The 2nd generation fluoroquinolones are effective in the treatment of prostatitis due to after oral administration of these drugs produce the highest concentration in prostatic fluid and prostatic tissue. Fluoroquinolones are effective in patients not responding to trimethoprim- sulfamethoxazole.[21]
-
STDs
A single oral dose of fluoroquinolones such as ofloxacin or ciprofloxacin is effective treatment for sensitive strains of N. gonorrhoeae due to its good efficacy. Sparfloxacin is an alternative treatment with doxycycline or a single dose of azithromycin, but increasing resistance to fluoroquinolones has led to ceftriaxone being the first- line agent for this infection.
-
Gastrointestinal and abdominal infections
The quinolones are equal to trimethoprim- sulfamethoxazole in effectiveness for traveler’s diarrhea mainly caused by Escherichia coli.[22] Norfloxacin, ciprofloxacin, and ofloxacin have been effective in the treatment of patients with shigellosis, enteric fever caused by S. typhi, as well as non-typhoidal infections in AIDS patients. Shigellosis is treated effectively with either ciprofloxacin or azithromycin.[23]
-
RTI
The key restriction to the use of quinolones for the treatment of community-acquired pneumonia and bronchitis had been observed because of poor in vitro activity of ciprofloxacin, ofloxacin, and norfloxacin against S. pneumoniae and anaerobic bacteria. Many newer fluoroquinolones, including gatifloxacin and moxifloxacin, have excellent activity against S. pneumoniae. These newer quinolones show comparable efficacy to β-lactam antibiotics.[24]
Bone, joint, and soft-tissue infections The treatment of chronic osteomyelitis requires prolonged antimicrobial therapy with agents active against S. aureus and Gram-negative rods. The fluoroquinolones may be used appropriately in some cases.[25]
Contraindications
Fluoroquinolones contraindicated in arrhythmic patient, not given to pregnant and lactating women because these drugs enter into breast milk and also cause the cartilage lesions, when used in children.[26]
Side effects
Central nervous system effects: Mild headache, drowsiness, insomnia, dizziness, mood alteration, peripheral neuropathy.
Chorionic villus sampling effects: Arrhythmia (prolongation of QT interval)
GIT effects: Diarrhea, abdominal pain, gastrointestinal irritation.
Hepatic toxicity, Genetic toxicity, and photosensitivity.[27,28]
Current prospective and future directions
At present, these drugs not use clinically due to bacterial resistance of fluoroquinolones at molecular level by different mechanisms. The future directions of fluoroquinolones are on nucleus that may be valuable target site to increase the potency, efficacy, and decrease side effects of fluoroquinolones.
CONCLUSION
The new fluoroquinolones show greatest activity against Gram-negative bacilli and improved Gram-positive activity. Ciprofloxacin still maintains the best in vitro activity against P. aeruginosa. Gatifloxacin, moxifloxacin, sparfloxacin, and trovafloxacin display improved activity against anaerobes versus ciprofloxacin. All of the new fluoroquinolones display excellent bioavailability and have longer serum half-lives than ciprofloxacin allowing for once daily dose administration. Prudent use of the new fluoroquinolones will be required to minimize the development of resistance to these agents.
Acknowledgment
We are grateful for all the respondents who contributed in this study.
Declaration of patient consent
Patient’s consent not required as patients identiy is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The fluoroquinolone antibacterials: Past, present and future perspectives. Int J Antimicrob Agents. 2000;16:5-15.
- [CrossRef] [Google Scholar]
- Quinolone In: Burgerís Medicinal Chemistry Drug Discovery. Hoboken, New Jersey: John Wiley and Sons; 2003. p. :582-7.
- [Google Scholar]
- Trimethoprim/ sulfamethoxazole resistance in urinary tract infections. J Emerg Med. 2009;36:338-41.
- [CrossRef] [PubMed] [Google Scholar]
- Expanded activity and utility of the new fluoroquinolones: A review. Clin Ther. 1999;21:3-40.
- [CrossRef] [Google Scholar]
- Quinolones In: Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver (2nd ed). Philadelphia, PA: Lippincott; 1999. p. :603.
- [Google Scholar]
- Antibacterial agents In: An Introduction to Medicinal Chemistry. Oxford, New York: Oxford University Press; 2003. p. :379-435.
- [Google Scholar]
- Synthesis and antitubercular activity of lipophilic moxifloxacin and gatifloxacin derivatives. Bioorg Med Chem Lett. 2007;17:5661-4.
- [CrossRef] [PubMed] [Google Scholar]
- Design, synthesis and activity against Toxoplasma gondii, Plasmodium spp. and Mycobacterium tuberculosis of new 6-fluoroquinolones. Eur J Med Chem. 2006;41:1478-93.
- [CrossRef] [PubMed] [Google Scholar]
- New classification and update on the quinolone antibiotics. Am Fam Physician. 2000;61:2741-8.
- [Google Scholar]
- A critical review of the fluoroquinolones: Focus on respiratory infections. Drugs. 2002;62:13-59.
- [CrossRef] [PubMed] [Google Scholar]
- Possible role for the new fluoroquinolones (levofloxacin, grepafloxacin, trovafloxacin, clinafloxacin, sparfloxacin, and DU-6859a) in the treatment of anaerobic infections: Review of current information on efficacy and safety. Clin Infect Dis. 1996;23:S25-30.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the effects of fourth-generation fluoroquinolones on corneal re-epithelialization in rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 2008;246:1455.
- [CrossRef] [PubMed] [Google Scholar]
- Design sunthesis and antimycobacterial activities of 1-methyl-2-alkenyl-4(1H)-quinolones. Bioorg Med Chem. 2011;19:567-79.
- [CrossRef] [PubMed] [Google Scholar]
- Interaction of N-methyl-2-alkenyl-4-quinolones with ATP dependent MurE ligase of Mycobacterium tuberculosis: Antibacterial activity molecular docking and inhibition kinetics. J Antimicrob Chemother. 2011;66:1766-72.
- [CrossRef] [PubMed] [Google Scholar]
- 1,8-naphthyridine derivatives. A new class of chemotherapeutic agents. J Med Pharm Chem. 1962;91:1063-5.
- [CrossRef] [PubMed] [Google Scholar]
- DNA gyrase and the supercoiling of DNA. Science. 1980;207:953-60.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin (BAY 12-8039) MICs and mutations in grlA, grlB, gyrA and gyrB in 116 unrelated clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 1998;41:481.
- [CrossRef] [PubMed] [Google Scholar]
- Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob Agents Chemother. 1991;35:1647-50.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of resistance to fluoroquinolones: State-of-the-art 1992-1994. Drugs. 1995;49(Suppl 2):29-35.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986;29:639-44.
- [CrossRef] [PubMed] [Google Scholar]
- Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:410-2.
- [CrossRef] [PubMed] [Google Scholar]
- Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-94.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention and treatment of traveler's diarrhea. N Engl J Med. 1993;328:1821-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of shigellosis: III Comparison of one-or two-dose ciprofloxacin with standard 5-day therapy a randomized blinded trial. Ann Intern Med. 1992;117:727-34.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin a double-blind randomized controlled trial. Ann Intern Med. 1997;126:697-703.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada Canadian bacterial surveillance network. N Engl J Med. 1999;341:233-9.
- [CrossRef] [PubMed] [Google Scholar]
- Safety of fluoroquinolones: An update. Can J Infect Dis. 2002;13:54-61.
- [CrossRef] [PubMed] [Google Scholar]







