Translate this page into:
Burden of carbapenem-resistant Acinetobacter baumannii involved in ventilator-associated pneumonia

*Corresponding author: Dr. Manita Paneri, Faculty of Health and Allied Sciences, Guru Kashi University, Bathinda, Punjab, India manitaprashant@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Paneri M, Saini RG, Singh M, Saini AK. Burden of carbapenem-resistant Acinetobacter baumannii involved in ventilator-associated pneumonia. Adesh Univ J Med Sci Res. doi: 10.25259/AUJMSR_3_2025
Abstract
Objectives:
Ventilator-associated pneumonia (VAP) is the most prevalent nosocomial infection of the intensive care unit (ICU) of any hospital, which accounts for more than 25% of all ICU infections. Among the Gram-negative bacilli, carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii are highlighted as “pathogens of concern” by the World Health Organization. Although carbapenems were formerly thought to be the backbone of treatment for life-threatening infections, these bacteria are rapidly acquiring resistance to carbapenems. Hence, the aim of this investigation was to identify and ascertain the prevalence of carbapenem-resistant A. baumannii from endotracheal aspirate (ETA) and bronchoalveolar lavage (BAL) samples, as well as the antibiotic susceptibility profile of this pathogen using VITEK 2 system.
Material and Method:
This prospective study was carried out from March 2023 to July 2024 at the Center for Interdisciplinary Biomedical Research, Adesh University, Bathinda. Two hundred suspected ETA and BAL samples were collected from the bacteriology lab of Adesh Hospital, Bathinda, Punjab. Identification and antibiotic susceptibility test for A. baumannii isolates were done by the VITEK 2 system.
Results:
Out of 200 VAP suspected endotracheal (ET)/BAL samples, a total 27 non-fermenters were isolated. Among 27 non-fermenters, A. baumannii was found in higher frequency (n = 17; 62.96%). In ET samples, its proportion was 66.66%, whereas in BAL samples, it was found to be only 33.33%. Among seventeen A. baumannii isolates, only two isolates (11.76%) were found susceptible to cefepime, ceftazidime, cefoperazone/sulbactam, piperacillin/tazobactam, meropenem, imipenem, gentamicin, ciprofloxacin, levofloxacin, and amikacin. Whereas, 88.23% isolates were resistant to all these drugs. Among all the antimicrobials, minocycline was found to be effective against A. baumannii as 82.35% isolates were found susceptible to it whereas, only 17.64% isolates were found to be resistant. Trimethoprim/sulfamethoxazole was effective against 47.05% isolates, whereas 52.94% isolates were resistant to it.
Conclusion:
Although in the present study, a low prevalence (8.5%) of A. baumannii causing VAP was seen due to effective infection control measures implemented by the infection control committee of the Adesh Hospital, Bathinda, but almost all strains were found to be carbapenem resistant, which is a worrisome situation. Thus, an effective infection control, including an interdisciplinary approach with microbiologists, is required to implement resistance surveillance in ICUs. The present study emphasizes the relevance of strictly adhering to “reserve antibiotics,” so carbapenems should be strictly avoided as an empirical treatment. Patients who are found positive for carbapenem-resistant pathogens should be isolated to avoid the dissemination. Antibiotic stewardship and awareness programs should be implemented by various stakeholders.
Keywords
Acinetobacter baumannii
Antibiotic resistance
Carbapenem
Pneumonia
Ventilator-associated pneumonia
INTRODUCTION
Ventilator-associated pneumonia (VAP) is defined as nosocomial pneumonia in patients who have been on ventilator support through an endotracheal (ET) tube or a tracheostomy for more than 48 h during intensive care unit (ICU) stay, excluding microbes that were present or incubating at the time of ICU admission.[1] The most significant class of antibiotics that is recommended as the first line of treatment for VAP patients is carbapenems.[2] Since carbapenems inhibit peptidoglycan cross-linking, causing cell lysis and death, they are regarded as bactericidal agents. However, the misjudiciary use of carbapenems leads to the emergence of Gram-negative bacteria that are resistant to them.[3]
Among the Gram-negative bacilli, carbapenem-resistant non-fermenters Pseudomonas aeruginosa and Acinetobacter baumannii both are highlighted as “pathogens of concern” by the World Health Organization.[4,5] Both are an opportunistic pathogen that are generally inhabited the hospital environment. By producing biofilm, acquiring and overexpressing various resistance genes, they can survive in harsh conditions, and typically infect only those patients who are mechanically ventilated or immunocompromised.[6,7] The most recent data from the Indian Council of Medical Research Antimicrobial Resistance surveillance network state that 80% of A. baumannii isolates were found imipenem-resistant.
Based on this, the present study was focused on the following objectives:
Phenotypic identification and characterization of A. baumannii involved in VAP by VITEK 2
Assessment of the antimicrobial susceptibility profile of the A. baumannii by VITEK 2.
MATERIAL AND METHODS
Ethical approval
This research work was carried out after obtaining approval from the Institutional Research Committee, followed by the Ethics Committee for Biomedical and Health Research, Adesh University (reference no. AU/DAA/02/2023/FA/405).
Two hundred suspected samples for VAP were received from the Bacteriology laboratory of the Department of Microbiology, Adesh Institute of Medical Sciences and Research, Bathinda. The sample size was calculated using Daniel formula.
Inclusion criteria
All ET aspirate/bronchoalveolar lavage (ETA/BAL) samples of suspected VAP patients, who were on mechanical ventilation for a period of more than 48 h were included in the present study.
Exclusion criteria
ETA/BAL samples of suspected VAP patients, with pneumonia before mechanical ventilation or before 48 h of mechanical ventilation were excluded from this study.
Sample collection
The ET/BAL samples from suspected VAP patients who were on mechanical ventilation support from >48 h, were collected from the Bacteriology laboratory of the Department of Microbiology, AIMSR, Bathinda. The samples were received by the laboratory from various ICUs of Adesh Hospital, Bathinda. An informed consent was taken from the patient’s relatives in English and local language as they were in an unconscious state.
Sample processing and bacterial isolation
After receiving ETA/BAL samples, these were divided into two parts. One part was preserved at −20℃ till further processing for deoxyribonucleic acid extraction. Second part was inoculated on blood agar and MacConkey agar plates. Plates were incubated aerobically overnight at 37°C. The agar plates showing significant bacterial growth of ≥103 colony-forming units (mL) were considered significant. Identification of A. baumannii isolates was achieved by examining colony characteristics and Gram staining. The colonies of A. baumannii were further identified by VITEK 2. Antibiotic susceptibility testing (AST) was done by VITEK 2 according to Shah et al.,[8] 2022 as per clinical and laboratory standards institute guidelines 2021 and all of those were preserved in 50% glycerol at −20°C.
RESULTS
In the present study out of two hundred ET/BAL suspected samples, 17 samples (8.5%) were positive for A. baumannii isolates.
Microbiological characteristics of A. baumannii
Blood agar: On blood agar, A. baumannii isolates showed small, convex, opaque, and grayish-white non-hemolytic colonies.
MacConkey agar: On MacConkey agar, A. baumannii isolates showed small, convex, opaque, and creamy nonlactose fermenting colonies with pinkish tint.
Nutrient agar: On nutrient agar, A. baumannii isolates showed smooth round, convex, opaque, and grayish colonies.
Age and gender wise distribution of patients infected by A. baumannii
There were 17 A. baumannii isolates from suspected ET/BAL samples, in which seven samples were from female patients (41.17%), and ten samples were obtained from male patients (58.82%). Out of the seven female patients, BAL sample was collected from only 1 patient (14.28%), ET samples were received from 6 female patients (85.71%), whereas in the male group, all ten ET samples were obtained from male patients (100%).
Table 1 shows percentage-wise ratio of males and female infected with A. baumannii according to different age group interval. Highest number of patients (58.82%) were seen in the age group between 51 and 75 years, whereas least were seen in the age group between 76 and 100 years, followed by 0–25 years (11.76%). Among the 17 patients infected with A. baumannii, there were 7 female patients (41.17%) and 10 male patients (58.82%). In the age group interval between 0 and 25 years, 2 female patients (28.57%), out of seven female patients, were infected with A. baumannii, whereas in age group between 51 and 75 years, 5 female patients (71.42%) were found to be infected with A. baumannii. In age groups 26–50 years, and 56–100 years, no female patient was seen infected with it. Among ten male patients, in the age group between 0 and 25 years, no male patient was suspected, in the age group 26–50 years, 4 male patients (40%) out of ten were infected with A. baumannii. In the age group 56–75 years, 5 male patients (50%) were infected with A. baumannii. In the age group between 76 and 100 years, only one male patient was suspected. These results indicate that patients in the age group 51–75 years are more prone to get A. baumannii infections in both sexes [Figure 1].
| Age-group (in years) | Female (n=7)(%) | Male (n=10) (%) | Total (n=17) |
|---|---|---|---|
| 0–25 | 2 (28.57) | 0 | 2 (11.76%) |
| 26–50 | 0 | 4 (40) | 4 (23.52%) |
| 51–75 | 5 (71.42) | 5 (50) | 10 (58.82%) |
| 76–100 | 0 | 1 (10) | 1 (5.88%) |
| Total | 7 (41.17) | 10 (58.82) | 17 |
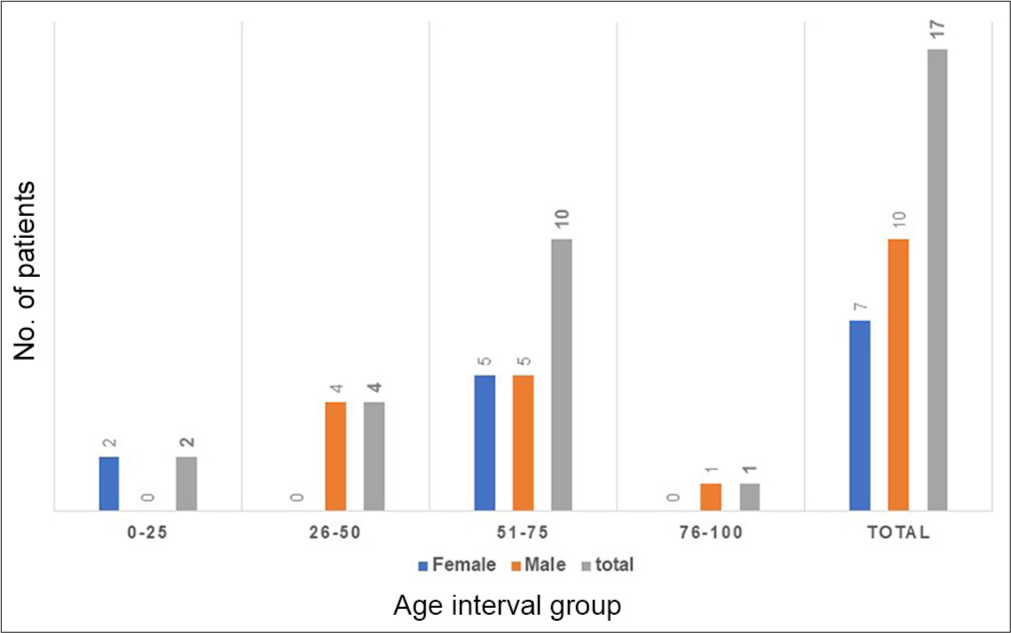
- Age group-wise distribution of ventilator-associated pneumonia patients infected with Acinetobacter baumannii.
AST result (A. baumannii n = 17)
The pattern of AST of 17 A. baumannii is given in Table 2.
| Antimicrobials | No. of susceptible isolates (%) | No. of resistant isolates (%) |
|---|---|---|
| Ceftazidime | 2 (11.76) | 15 (88.23) |
| Cefepime | 2 (11.76) | 15 (88.23) |
| Cefoperazone/sulbactam | 2 (11.76) | 15 (88.23) |
| Piperacillin/tazobactam | 2 (11.76) | 15 (88.23) |
| Imipenem | 2 (11.76) | 15 (88.23) |
| Meropenem | 2 (11.76) | 15 (88.23) |
| Amikacin | 2 (11.76) | 15 (88.23) |
| Gentamicin | 2 (11.76) | 15 (88.23) |
| Ciprofloxacin | 2 (11.76) | 15 (88.23) |
| Levofloxacin | 2 (11.76) | 15 (88.23) |
| Trimethoprim/sulfamethoxazole | 8 (47.05) | 9 (52.94) |
| Minocycline | 14 (82.35) | 3 (17.64) |
Among 17 A. baumannii isolates, only 2 isolates (11.76%) were susceptible to cefepime, ceftazidime, cefoperazone/sulbactam, piperacillin/tazobactam, meropenem, imipenem, gentamicin, ciprofloxacin, levofloxacin, trimethoprim/sulfamethoxazole, and amikacin. Whereas, 15 isolates (88.23%) were resistant to all these drugs.
Among all the antimicrobials, minocycline was found to be most effective against A. baumannii isolates as 82.35% isolates were found susceptible to it and only 17.64% isolates of were resistant. Trimethoprim/sulfamethoxazole was effective against 47.05% isolates, whereas 52.94% isolates were resistant to it. All the other antimicrobials were not effective against A. baumannii.
DISCUSSION
VAP is the most prevalent nosocomial infection in the ICU, especially in immunocompromised patients requiring mechanical ventilation for extended periods of time.[9] Patients who are older and immunosensecence are more vulnerable to VAP, due to lowered antibody responses, decreased T-cell function, and dysregulated inflammatory responses.[10,11] A. baumannii, P. aeruginosa, and Stenotrophomonas maltophilia are among the carbapenem-resistant non-fermenting Gram-negative bacilli that have caused a significant increase in VAP burden in India in the preceding 5 years.[12,13] According to Alnimr (2023), the mortality rate for VAP brought on by carbapenem-resistant A. baumannii (CRAB) strains was more than 50%, which happens to be much greater than the mortality rate of strains susceptible to this antibiotic.[14] Hospitals frequently get endemic, due to the multidrug-resistant A. baumannii. Porins, capsules, as well as proteins are a few of the virulence factors involved in pathogenesis.[15] By virtue of its resilience, A. baumannii can withstand hostile environments, which can result in serious infections. Because A. baumannii has a unique ability to retain a multidrug-resistant phenotype, major antibiotic classes become less efficient.[16] Eight to fourteen percentages of VAP in the US and Europe are caused A. baumannii ; however, in Asia, Latin America, and several Middle Eastern countries, this bacterium is linked to greater rates (19–>50%) according to Ayoub Moubareck and Hammoudi Halat (2020).[17] Saleem et al., 2022 in their 2 years prospective study, reported high prevalence of XDR A. baumannii (9%) involved in VAP.[18]
In the present study, A. baumannii isolates were found to be resistant against almost all the 12 antibiotics. Similar findings were reported by Kumari et al., 2021.[19] Maximum effective drug was minocycline. In this study, almost 82.35% isolates were susceptible to this antibiotic, whereas Yang et al. (2022) reported that 55% carbapenem isolates were susceptible to minocycline.[20] Beheshti et al. (2020) mentioned in their findings that 71% A. baumannii isolates were susceptible to minocycline.[21] Srivastava et al. (2024) demonstrated that minocycline is more effective antimicrobial than beta-lactam antibiotics.[22] Adukauskiene et al. (2022) reported that almost 90% A. baumannii strains were resistant to carbapenem, cephalosporin, and piperacillin/tazobactam.[9] These findings are similar to the results obtained in the present study where more than 88.23% isolates were resistant to these antimicrobials. Sharma et al. (2022) demonstrated that more than 90% A. baumannii isolates were resistant to ceftazidime, cefepime, levofloxacin, ciprofloxacin, cotrimoxazole, and piperacillin/tazobactam.[23]
A unique outer membrane protein called OprD, which permits the antibiotic to enter the cell, has been lost in A. baumannii making it resistant to carbapenem antibiotics A. baumannii is capable of producing changes in the structure of bacterial cells that hinder the ability of the antibiotic to fuse to its target and carbapenemases, which degrade carbapenem drugs. Minocycline is a type of tetracycline that is able to bind with bacterial ribosomes which, thus, inhibit protein synthesis.[20] It may produce antimicrobial peptides and inhibit the formation of bacterial cell wall. Its lengthy half-life, low minimum inhibitory concentration, and high concentration within tissues are among its pharmacodynamic characteristics. The evidence supporting minocycline’s efficacy against CRAB is, however, weak, and more research is required to validate its efficacy, track resistance, and ascertain its usefulness in combination therapy.[8,24]
CONCLUSION
Although in the present study, a low prevalence (8.5%) of A. baumannii causing VAP was seen due to effective infection control measures implemented by the infection control committee of the Adesh Hospital, Bathinda, almost all strains were found to be carbapenem resistant, which is a worrisome situation. Thus, an effective infection control, including an interdisciplinary approach with microbiologists, is required to implement resistance surveillance in ICUs. The present study emphasizes the relevance of strictly adhering to “reserve antibiotics,” so carbapenems should be strictly avoided as an empirical treatment. Patients who are found positive for carbapenem-resistant pathogens should be isolated to avoid the dissemination. Antibiotic stewardship and awareness programs should be implemented by various stakeholders.
Acknowledgment:
The authors thank Dr. Upasana Bumbla (Professor and Head of the Department, Microbiology, Adesh Institute of Medical Sciences and Research) and her team, and Dr. Avneet Garg (Department of Respiratory Medicine) and for their support.
Ethical approval:
The research/study approved by the Institutional Review Board at Adesh University, number AU/DAA/02/2023/FA/405, dated 12th February 2023.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
The abstract was submitted for oral presentation at the “2nd International Conference on Clinical and Applied Microbiology” organized by BioLEAGUES on the 14th and 15th December 2023.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Evaluating antibiotic therapy for ventilator-associated pneumonia caused by gram-negative bacilli. Surg Open Sci. 2023;16:64-7.
- [CrossRef] [PubMed] [Google Scholar]
- Carbapenem-resistant gram-negative bacilli causing ventilator associated pneumonia: Study of MASTDISCS combi carba plus for detection of carbapenemase producing enterobacterales. Infect Drug Resist. 2022;15:6331-42.
- [CrossRef] [PubMed] [Google Scholar]
- Overview of antimicrobial resistance: An emerging silent pandemic. Global J Med Pharm Biomed Update. 2023;18:11.
- [CrossRef] [Google Scholar]
- Molecular analysis of carbapenem and aminoglycoside resistance genes in carbapenem-resistant Pseudomonas aeruginosa clinical strains: A challenge for tertiary care hospitals. Antibiotics. 2024;13:191.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of carbapenem-resistant gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis. 2017;4:ofx176.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of carbapenem resistant Acinetobacter baumannii harboring blaOXA genes in the Indian intensive care unit. Global J Med Pharm Biomed Update. 2023;18:12.
- [CrossRef] [Google Scholar]
- Detection of antimicrobial resistance genes associated with carbapenem resistance from the whole-genome sequence of Acinetobacter baumannii isolates from Malaysia. Can J Infect Dis Med Microbiol. 2020;2020:5021064.
- [CrossRef] [PubMed] [Google Scholar]
- Bacteriological profile and antibiotic susceptibility pattern of tracheal secretions isolates among intensive care unit patients at tertiary care hospital. CHRISMED J Health Research. 2022;9:262-7.
- [CrossRef] [Google Scholar]
- Clinical features and outcomes of monobacterial and polybacterial episodes of ventilator-associated pneumonia due to multidrug-resistant Acinetobacter baumannii. Antibiotics. 2022;11:892.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation on risk factors of ventilator-associated pneumonia in acute cerebral haemorrhage patients in intensive care unit. Can Respir J. 2017;2017:7272080.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and microbiologic efficacy and safety of imipenem/cilastatin/relebactam in complicated infections: A meta-analysis. Infect Chemother. 2021;53:271-83.
- [CrossRef] [PubMed] [Google Scholar]
- Novel B-lactam-B-lactamase inhibitor combinations: Expectations for the treatment of carbapenem-resistant gram-negative pathogens. Expert Opin Drug Metab Toxicol. 2019;15:133-49.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial resistance in ventilator-associated pneumonia: Predictive microbiology and evidence-based therapy. Infect Dis Ther. 2023;12:1527-52.
- [CrossRef] [PubMed] [Google Scholar]
- Infections due to Acinetobacter baumannii in the ICU: Treatment options. Semin Respir Crit Care Med. 2017;38:311-25.
- [CrossRef] [PubMed] [Google Scholar]
- Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur J Pharm Sci. 2019;136:104940.
- [CrossRef] [PubMed] [Google Scholar]
- Insights into Acinetobacter baumannii A Review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics (Basel, Switzerland). 2020;9:119.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular characterization and antibiogram of Acinetobacter baumannii clinical isolates recovered from the patients with ventilator-associated pneumonia. Healthcare. 2022;10:2210.
- [CrossRef] [PubMed] [Google Scholar]
- Emergence of blandm-1 and blavim producing gram-negative bacilli in ventilator-associated pneumonia at AMR surveillance regional reference laboratory in India. PLoS One. 2021;16:E0256308.
- [CrossRef] [PubMed] [Google Scholar]
- Minocycline susceptibility and Tetb gene in carbapenem-resistant Acinetobacter baumannii in Taiwan. Infect Drug Resist. 2022;15:2401-8.
- [CrossRef] [PubMed] [Google Scholar]
- Tetracycline resistance mediated by Tet efflux pumps in clinical isolates of Acinetobacter baumannii. Rev Inst Med Trop São Paulo. 2020;62:E88.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-microbiological profile of Acinetobacter baumannii infections in a tertiary care hospital in Greater Noida. Int J Acad Med Pharm. 2024;6:517-20.
- [Google Scholar]
- Isolation of non-fermenting gram-negative bacteria in respiratory tract infections. IP Int J Med Microbiol Trop Dis. 2020;6:184-7.
- [CrossRef] [Google Scholar]
- Minocycline for the treatment of multidrug and extensively drug-resistant A. baumannii A review. Infect Dis Ther. 2017;6:199-211.
- [CrossRef] [PubMed] [Google Scholar]








