Translate this page into:
Anterior communicating artery aneurysm microsurgery resulted in a proximal pseudoaneurysm

*Corresponding author: Mustafa Ismail, Department of Surgery, Baghdad Teaching Hospital, Medical City Complex, Baghdad, Iraq. mustafalorance2233@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ismail M, Hummadi NA, Al-Waely NK, Albanaa SA, Neamah AM, Al-Khafaji AO, et al. Anterior communicating artery aneurysm microsurgery resulted in a proximal pseudoaneurysm. Adesh Univ J Med Sci Res 2023;5:84-7. doi: 10.25259/AUJMSR_21_2023
Abstract
Intracranial pseudoaneurysms (IPAs), characterized by compromised vessel wall layers, represent <1% of all intracranial aneurysms. They often materialize along the vessel wall and are vulnerable to rupture, resulting in a mortality rate of up to 50%. Treatment is complex due to the fragility of pseudoaneurysms, the absence of a suitable surgical neck, and frequently associated intracranial atherosclerosis and calcification. This study reports a rare instance of an IPA following a microsurgical clipping of an intracranial aneurysm. A 48-year-old male with severe headache, vomiting, and brief unconsciousness was diagnosed with a ruptured anterior communicating artery aneurysm. After successful microsurgical clipping, a follow-up computed tomography angiogram revealed a new aneurysmal dilation on the anterior aspect of the pre-bifurcation segment of the right internal carotid artery (ICA). A subsequent craniotomy was performed to address the ICA pseudoaneurysm. Owing to the calcification surrounding the pseudoaneurysm, it was safeguarded with crushed muscle and cellulose fabric. Follow-ups showed no pseudoaneurysm enlargement, and the patient recovered with no neurological deficits. Iatrogenic IPAs post-aneurysmal clipping are exceptionally rare yet dangerous entities necessitating rapid intervention to minimize morbidity and mortality. Tailoring treatment modalities based on patient-specific characteristics, lesion attributes, and surgical expertise is fundamental. A cautious follow-up regimen is also suggested to monitor the pseudoaneurysm’s dimensions effectively.
Keywords
Pseudoaneurysm
Iatrogenic
Microsurgical clipping
Intracranial aneurysm
Anterior communicating artery
INTRODUCTION
Intracranial pseudoaneurysms (IPAs), a type of false aneurysm distinguished by its pathological anatomy, are infrequent yet perilous cerebrovascular anomalies. These formations are often not identified until they rupture. The distinguishing feature of IPAs lies in the compromise of all three layers of the vessel wall, resulting in a swift accumulation of an external blood clot, causing vessel dilation. IPAs constitute <1% of all intracranial aneurysms and are especially common among pediatric patients.[1,2]
A majority of IPAs result from indirect blunt head trauma, although additional causes include direct vascular trauma, either iatrogenic or otherwise, and conditions such as Marfan’s syndrome, fibromuscular dysplasia, and certain infections or vasculitides.[2,3] Unlike true intracranial aneurysms, which tend to develop at bifurcation points, IPAs usually materialize along the vessel wall, distant from bifurcation sites. Due to the delicate nature of their thin walls and insufficient support, IPAs are susceptible to rupture, a scenario that carries a mortality rate of up to 50%.[4] Moreover, the treatment of IPAs presents significant challenges owing to the fragility of pseudoaneurysms, the absence of a suitable surgical neck, the common coexistence of intracranial atherosclerosis, and calcification in the surrounding area. Various treatment methods have been documented, but none have demonstrated unequivocal superiority. Therefore, strategies for IPA management tend to be determined by factors specific to the patient, lesion characteristics, and the surgeon’s expertise.
IPAs can occur as a consequence of endovascular coiling of true aneurysms, a medical intervention. On rare occasions, IPAs develop following open surgery due to unintended vascular damage during tumor excision procedures. Such occurrence is highly uncommon post-microsurgical clipping of intracranial aneurysms.[5] To our knowledge, this report documents only the fourth instance of an iatrogenic pseudoaneurysm appearing after microsurgical clipping of an intracranial aneurysm. It also marks the first-ever documentation of an IPA in the supraclinoid internal carotid artery (ICA) following successful microsurgical clipping of an anterior communicating artery (Acom) aneurysm.
CASE REPORT
A 48-year-old male presented himself at the Neurosurgical Teaching Hospital in Baghdad, Iraq, suffering from a sudden and severe headache accompanied by vomiting and a brief unconscious spell. The clinical examination did not yield any remarkable findings, except for neck stiffness, with the Glasgow Coma Scale score of the patient standing at 14 (E3V5M6). Cranial computed tomography (CT) disclosed a diffuse subarachnoid hemorrhage in the basal cisterns. Further investigation through a CT angiogram (CTA) unveiled an Acom aneurysm [Figure 1]. In light of the unavailability of endovascular facilities, catheter angiography was not carried out.
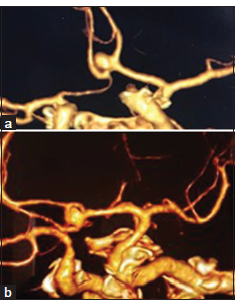
- (a) Pre-operative anterior view of cerebral computed tomography angiogram (CTA) (3D reconstruction) shows a right superiorly-projecting anterior communicating artery (Acom) aneurysm. (b) Pre-operative left lateral view of cerebral CTA (3D reconstruction) demonstrates a right superiorly projecting Acom aneurysm.
In response to the ruptured state of the aneurysm, its positioning at the Acom, and its wide neck, the patient underwent a microsurgical clipping procedure using a right lateral supraorbital approach. During the operation, after the temporary cessation of blood flow in the A1 segment of the anterior cerebral artery (ACA), the aneurysm’s neck was successfully clipped. This preserved the post-communicating segments of the ACA on both sides. A comprehensive inspection of the region revealed no collateral damage to surrounding structures, and there was no rupture of the aneurysm during surgery. The procedure was completed smoothly with no perioperative complications.
A CTA, conducted 7-day post-surgery, verified the successful clipping of the aneurysmal neck and the absence of aneurysmal remnants. However, it did reveal an aneurysmal dilation, measuring 3 mm, on the anterior aspect of the prebifurcation segment of the right ICA, a feature absent in earlier scans [Figure 2]. Post-procedure consultation led to a subsequently delayed craniotomy, performed a month later, to address the newfound ICA aneurysm.
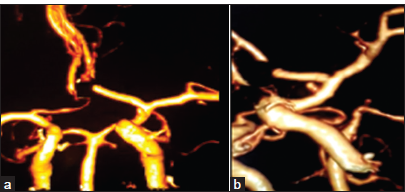
- (a) Post-operative anterior view of computed tomography angiogram (CTA) shows complete clipping of the anterior communicating artery aneurysm with preservation of A2 segments bilaterally and no aneurysmal remnants and, a small anteriorly projecting pseudoaneurysm arising from the anterior wall of the pre-bifurcation internal carotid artery (ICA). (b) Post-operative left lateral view of CTA affirms the surgical clipping of the aneurysm and the presence of a small anteriorly-projecting pseudoaneurysm arising from the anterior wall of the pre-bifurcation ICA.
During this second operation, the immediate area surrounding the pseudoaneurysm was found to be calcified, posing significant difficulties in dissection and clipping of the pseudoaneurysm without triggering a rupture. As a result, the aneurysm was safeguarded using a strip of crushed muscle and cellulose fabric wrapping. Follow-up CTAs conducted post-operation and at the 6-month mark showed no enlargement of the pseudoaneurysm [Figure 3]. The patient continued to recover well with no neurological deficiencies, and a semiannual follow-up regimen using magnetic resonance angiography was recommended to monitor the aneurysm’s dimensions.
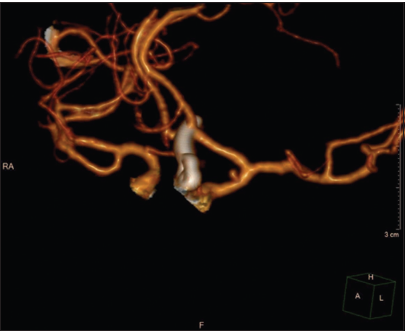
- Follow-up computed tomography angiogram 1 month after the second surgery showing complete clipping of the anterior communicating artery aneurysm as well as persistence of the internal carotid artery pseudoaneurysm without any change in size.
DISCUSSION
Iatrogenic IPAs arise as a consequence of inadvertent arterial damage during either endovascular or surgical interventions, typically during skull base tumor excisions. This damage precipitates the formation of two morphological forms of pseudoaneurysms: Fusiform and saccular.[5] The primary location for IPA formation is within the ICA, with certain factors increasing susceptibility to IPA formation, including anatomical variations of skull base bones (especially the sphenoid and temporal bones), tumor invasion into the cavernous sinus or ICA, and an irregular course of the ICA. IPA formation can also occur in other locations such as the ACA, Acom, posterior cerebral artery, posterior inferior cerebellar artery, superior cerebellar artery (SCA), basilar artery, and other intracranial arteries.[6]
In our presented case, an Acom aneurysm microsurgical clipping operation led to the secondary development of a small pseudoaneurysm on the anterior wall of the prebifurcation ICA. Iatrogenic IPA formation at the site of clipped intracranial aneurysms has been documented in the past.[4] Nevertheless, the manifestation of a pseudoaneurysm in an artery distinct from the one that underwent microsurgical clipping is exceptionally rare and has only been reported once.[7] Rowed and Walters[7] recorded a case of a supraclinoid ICA pseudoaneurysm that was detected 8 days after a minor ICA trauma during the microsurgical clipping of an SCA aneurysm. This IPA formation was ascribed to minor ICA trauma induced by traction on the posterior communicating artery, resulting in a small full-thickness ICA wall laceration and subsequent bleeding that ceased after applying brief pressure tamponade to the laceration.
Other suggested causes of iatrogenic IPA formation secondary to aneurysm clipping include minor direct trauma to the vessels, tearing of small perforating arteries, traction injury at bifurcation points, and temporary clipping-related injury.[6,7] In our case, we noted no apparent vessel wall injury upon careful vessel examination within the surgical corridor. Temporary clips were only placed on the A1 segment of the right ACA, distant from the ICA pseudoaneurysm site. However, minor ICA traction during the A1 manipulation could explain this new lesion’s formation. Furthermore, postoperative vasospasm has been reported to compromise the vasa vasorum of neighboring vessels, including the ICA. This could lead to regional ischemia, vessel wall weakening, and exacerbation of any minute tears.[6] This mechanism may also elucidate the formation of the IPA away from the clipping site as occurred in the present case.
Treatment options for IPAs span microsurgical clipping, endovascular treatment, and conservative management. The principal aim of the treatment is to negate the risk of rebleeding, which necessitates timely intervention, as most pseudoaneurysms bleed within 2–4 weeks of their formation.[6,7] Endovascular treatment can occlude the vascular or stent the affected artery. The selected endovascular technique is dependent on the IPA location; proximal lesions are good stent candidates, whereas more distal lesions due to their small caliber may be more susceptible to endovascular coiling or embolization. Endovascular options have shown variable results in IPAs treatment. Still, with continual advancements and the introduction of new endovascular tools, they are increasingly integrated into pseudoaneurysm management.[8]
The surgical treatment of cerebral pseudoaneurysms carries a significant risk of losing parental vessel patency. Hence, preserving adequate blood flow to the impacted vessel and its perforators are vital for positive surgical outcomes. In addition, surgical dissection around the pseudoaneurysm carries a considerable risk of aneurysmal rupture due to the fragility of the pseudoaneurysm wall, especially when accompanied by adhesions and calcification from previous surgeries. Consequently, surgical management of pseudoaneurysms poses a substantial risk of patient morbidity and must be tailored to each individual case. Surgical alternatives include clipping with a Sugita encircling clip, aneurysm wrapping with muscle, polytetrafluoroethylene or cellulose fabric, or a combination of clipping and wrapping. Recently, surgical trapping and/or resection of the pseudoaneurysm with extracranial-intracranial or intracranial-intracranial bypass has been demonstrated to be a safe and effective treatment strategy, with very favorable outcomes over a 19-month period.
In contrast, aneurysm resolution following conservative management is exceptionally rare.[9] However, it has proven successful in certain pseudoaneurysm cases.[10] Factors predicting a favorable outcome of conservative management include young age, reduction in lesion size and flow on subsequent imaging, and posterior circulation pseudoaneurysms, likely due to different flow dynamics within these lesions.[10]
Unlike ruptured true cerebral aneurysms, there are no large-scale studies on cerebral pseudoaneurysms to discern the superior treatment option, and it remains largely contingent on patient and lesion-related factors, as well as the surgeon’s experience.[9] In the current case, the pseudoaneurysm vicinity was found to be calcified and covered with rigid adhesions, and manipulation was considered too hazardous due to the pseudoaneurysm wall’s fragility and the high rupture risk. As a result, the aneurysm was secured and wrapped with muscle and cellulose fiber. This proved a successful treatment strategy, as the patient’s follow-up imaging displayed no pseudoaneurysm enlargement or signs of bleeding.
CONCLUSION
Iatrogenic IPAs emerging post-aneurysmal clipping procedures represent exceedingly rare pathologies associated with a significant likelihood of early hemorrhage. This mandates prompt intervention to avert potential morbidity and mortality. The choice of treatment ought to be customized, factoring in the unique attributes of both the patient and lesion, as well as the surgeon’s proficiency.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Intracranial vascular lesions associated with small epidural hematomas. Neurosurgery. 2008;62:416-21.
- [CrossRef] [PubMed] [Google Scholar]
- The international cooperative study on the timing of aneurysm surgery. J Neurosurg. 1990;37:18-36.
- [CrossRef] [PubMed] [Google Scholar]
- Traumatic intracranial carotid tree aneurysms. Neurosurgery. 1998;43:1314-20.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular management of ventricular catheter-induced anterior cerebral artery false aneurysm: Technical case report. Neurosurgery. 2005;57:E374.
- [CrossRef] [PubMed] [Google Scholar]
- Iatrogenic intracranial pseudoaneurysms: Neuroradiological and therapeutical considerations, including endovascular options. Neurol Sci. 2006;27:317-22.
- [CrossRef] [PubMed] [Google Scholar]
- Acute formation of a pseudoaneurysm adjacent to a previously clipped anterior communicating artery aneurysm. Surg Neurol Int. 2011;2:56.
- [CrossRef] [PubMed] [Google Scholar]
- Iatrogenic false aneurysm following repair of intracranial aneurysm. Can J Neurol Sci. 1994;21:346-9.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical strategies for ruptured blister-like aneurysms arising from the internal carotid artery: A clinical analysis of 18 consecutive patients. Acta Neurochir (Wien). 2009;151:125-30.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurol. 2009;8:427-33.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous resolution of perforator aneurysms of the posterior circulation. J Neurosurg. 2014;121:1107-11.
- [CrossRef] [PubMed] [Google Scholar]








